Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S9SJ
|
|||
| Former ID |
DIB020942
|
|||
| Drug Name |
SGC0946
|
|||
| Synonyms |
SGC0946; 1561178-17-3; SGC 0946; CHEMBL3087498; SGC-0946; 5-Bromo-7-{5-[(3-{[(4-Tert-Butylphenyl)carbamoyl]amino}propyl)(Propan-2-Yl)amino]-5-Deoxy-Beta-D-Ribofuranosyl}-7h-Pyrrolo[2,3-D]pyrimidin-4-Amine; 1-(3-((((2R,3S,4R,5R)-5-(4-Amino-5-bromo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(isopropyl)amino)propyl)-3-(4-(tert-butyl)phenyl)urea; MLS006011035; C28H40BrN7O4; GTPL7020; SCHEMBL17433345; AOB3554; EX-A321; MolPort-028-720-498; ZINC97956664; s7079; BDBM50443016; 2714AH; AKOS024458211
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
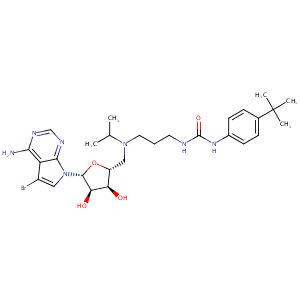 |
Download2D MOL
|
||
| Formula |
C28H40BrN7O4
|
|||
| Canonical SMILES |
CC(C)N(CCCNC(=O)NC1=CC=C(C=C1)C(C)(C)C)CC2C(C(C(O2)N3C=C(C4=C(N=CN=C43)N)Br)O)O
|
|||
| InChI |
1S/C28H40BrN7O4/c1-16(2)35(12-6-11-31-27(39)34-18-9-7-17(8-10-18)28(3,4)5)14-20-22(37)23(38)26(40-20)36-13-19(29)21-24(30)32-15-33-25(21)36/h7-10,13,15-16,20,22-23,26,37-38H,6,11-12,14H2,1-5H3,(H2,30,32,33)(H2,31,34,39)/t20-,22-,23-,26-/m1/s1
|
|||
| InChIKey |
IQCKJUKAQJINMK-HUBRGWSESA-N
|
|||
| CAS Number |
CAS 1561178-17-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histone-lysine N-methyltransferase (HLNM) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Lysine degradation | |||
| Transcriptional misregulation in cancer | ||||
| Reactome | PKMTs methylate histone lysines | |||
| WikiPathways | Histone Modifications | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Catalytic site remodelling of the DOT1L methyltransferase by selective inhibitors. Nat Commun. 2012;3:1288. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

