Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S4SX
|
|||
| Former ID |
DNCL001837
|
|||
| Drug Name |
K-134
|
|||
| Synonyms |
SULBACTAM; 68373-14-8; sulbactam acid; Sulbactamum; Betamaze; Penicillanic Acid Sulfone; (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide; penicillanic acid 1,1-dioxide; UNII-S4TF6I2330; CP 45899; CHEMBL403; CHEBI:9321; S4TF6I2330; Sulbactam;Penicillanic acid sulfone; (2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid 4,4-dioxide; Sulbactam [INN:BAN]; CP-45,899; Sulbactamum [INN-Latin]; DSSTox_CID_3605; DSSTox_RID_77104
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Arteriosclerosis [ICD-11: BD40; ICD-9: 440] | Phase 2 | [1] | |
| Company |
Kowa Pharmaceuticals America
|
|||
| Structure |
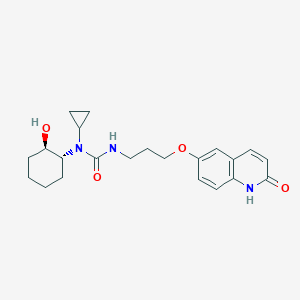 |
Download2D MOL |
||
| Formula |
C22H29N3O4
|
|||
| Canonical SMILES |
C1CCC(C(C1)N(C2CC2)C(=O)NCCCOC3=CC4=C(C=C3)NC(=O)C=C4)O
|
|||
| InChI |
1S/C22H29N3O4/c26-20-5-2-1-4-19(20)25(16-7-8-16)22(28)23-12-3-13-29-17-9-10-18-15(14-17)6-11-21(27)24-18/h6,9-11,14,16,19-20,26H,1-5,7-8,12-13H2,(H,23,28)(H,24,27)/t19-,20-/m1/s1
|
|||
| InChIKey |
ULGNGSQNNMKROG-WOJBJXKFSA-N
|
|||
| CAS Number |
CAS 189362-06-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9972, 618291, 7980696, 10242577, 14847263, 15121712, 25622048, 29215246, 29308945, 47193857, 48416564, 50085946, 50148676, 57269561, 57343357, 85279331, 90444204, 92309083, 92717113, 96025218, 103163858, 103932643, 104372503, 108667192, 117668018, 124766368, 124812411, 126622488, 127859141, 131323571, 134222618, 134350930, 135009448, 135692345, 135693785, 135727108, 136974559, 137022389, 137981775, 144187536, 144207191, 160811130, 162107598, 162178761, 162630505, 164196445, 164814570, 169618960, 170465248, 174006239
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 3 (PDE3) | Target Info | Modulator | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00783081) Safety and Efficacy of K-134 for the Treatment of Intermittent Claudication. U.S. National Institutes of Health. | |||
| REF 2 | A phase II dose-ranging study of the phosphodiesterase inhibitor K-134 in patients with peripheral artery disease and claudication. J Vasc Surg. 2012 Feb;55(2):381-389.e1. | |||
| REF 3 | K-134, a Phosphodiesterase 3 Inhibitor, Prevents Brain Damage by Inhibiting Thrombus Formation in a Rat Cerebral Infarction Model. PLoS One. 2012; 7(10): e46432. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

