Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S4JK
|
|||
| Former ID |
DCL000170
|
|||
| Drug Name |
MLN8237
|
|||
| Synonyms |
Alisertib
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 3 | [1], [2] | |
| Small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162.9] | Phase 2 | [3] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
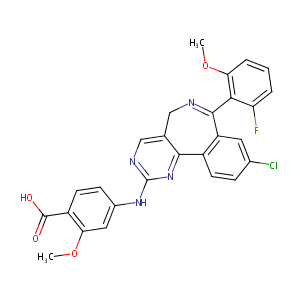 |
Download2D MOL |
||
| Formula |
C27H20ClFN4O4
|
|||
| Canonical SMILES |
COC1=C(C(=CC=C1)F)C2=NCC3=CN=C(N=C3C4=C2C=C(C=C4)Cl)NC5=CC(=C(C=C5)C(=O)O)OC
|
|||
| InChI |
1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33)
|
|||
| InChIKey |
ZLHFILGSQDJULK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1028486-01-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
49693830, 57107096, 99436914, 103651972, 103905632, 104120110, 123055410, 124757022, 125163826, 131477692, 131480698, 134223405, 134338845, 135263892, 135626804, 135727405, 136367323, 136920350, 137173580, 137276087, 141669651, 143499693, 144116244, 152064066, 152234949, 152258281, 152344273, 160647120, 160838255, 162011658, 162037455, 162202572, 163098799, 163403893, 164193986, 164339418, 172095365, 172918135, 174006392, 174525840, 180111921, 185998541, 188899555, 198972240, 198993425, 204398557, 223366121, 223382037, 223613049, 223705104
|
|||
| ChEBI ID |
CHEBI:125628
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aurora kinase A (AURKA) | Target Info | Inhibitor | [4] |
| KEGG Pathway | Oocyte meiosis | |||
| Pathway Interaction Database | Aurora B signaling | |||
| Signaling by Aurora kinases | ||||
| Integrin-linked kinase signaling | ||||
| PLK1 signaling events | ||||
| Aurora A signaling | ||||
| Reactome | APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 | |||
| Regulation of PLK1 Activity at G2/M Transition | ||||
| WikiPathways | EGF/EGFR Signaling Pathway | |||
| JAK/STAT | ||||
| Gastric Cancer Network 1 | ||||
| Integrated Breast Cancer Pathway | ||||
| APC/C-mediated degradation of cell cycle proteins | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7790). | |||
| REF 2 | ClinicalTrials.gov (NCT01482962) Alisertib (MLN8237) or Investigator's Choice in Patients With Relapsed/Refractory Peripheral T-Cell Lymphoma. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Effect of Aurora A kinase inhibitor MLN8237 combined with rituximab on antitumor activity in preclinical B-cell non-Hodgkin's lymphoma models. Journal of Clinical Oncology, 2009:8553. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

