Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0RE9C
|
|||
| Drug Name |
Lucerastat
|
|||
| Synonyms |
141206-42-0; UNII-GVS3YDM418; N-Butyldeoxygalactonojirimycin; GVS3YDM418; CHEMBL1086997; N-(n-Butyl)deoxygalactonojirimycin; (2R,3S,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol; Lucerastat [INN]; N-Butyl-DGJ; NB-DGJ; N-Bu-DGJ; AC1L9UXN; N- Butyldeoxygalactonojirimycin; SCHEMBL6821044; N-Butyl-deoxy-galactonojirimycin; CDP-923; N-N-Butyl Deoxygalactonojirimycin; OGT-923; DTXSID60161601; MolPort-039-015-418; ZX-AT009021; ZINC13719785; BDBM50312528; N-Butyl-D-galacto-1-deoxynojirimycin; AKOS027384398; NCGC00181326
Click to Show/Hide
|
|||
| Indication | Fabry disease [ICD-11: 5C56.01; ICD-9: 272.7] | Phase 3 | [1] | |
| Company |
Actelion Pharmaceuticals South San Francisco, CA
|
|||
| Structure |
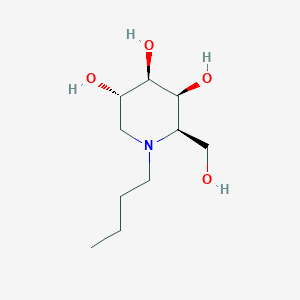 |
Download2D MOL |
||
| Formula |
C10H21NO4
|
|||
| Canonical SMILES |
CCCCN1CC(C(C(C1CO)O)O)O
|
|||
| InChI |
1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9+,10-/m1/s1
|
|||
| InChIKey |
UQRORFVVSGFNRO-XFWSIPNHSA-N
|
|||
| CAS Number |
CAS 141206-42-0
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03737214) A Study to Evaluate the Long-term Safety and Tolerability of Lucerastat in Adult Subjects With Fabry Disease. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

