Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R9BG
|
|||
| Former ID |
DAP001277
|
|||
| Drug Name |
Acetohydroxamic Acid
|
|||
| Synonyms |
AHA; Acethydroxamsaeure; Acethydroxamsaure; Acetohydroxamate; HAE; Lithostat; Acethydroxamic acid; Acethydroxamsaeure [German]; Acetohydroximic acid; Acetyl hydroxyamino; Acetylhydroxamic acid; Acide acetohydroxamique; Acide acetohydroxamique [French]; Acido acetohidroxamico; Acido acetohidroxamico [Spanish]; Acidum acetohydroxamicum; Acidum acetohydroxamicum [Latin]; Cetohyroxamic acid; Methylhydroxamic acid; SJX HLdmMAH; AHA (TN); Acetic acid, oxime; Acetohydroxamic acid [USAN:INN]; Lithostat (TN); N-Acetyl hydroxyacetamide; N-Acetylhydroxylamine; N-Hydroxyacetamide; N-hydroxyacetimidic acid; N-hydroxyethanimidic acid; S14-0751; Acetohydroxamic acid (USP/INN); Acetamide, N-hydroxy-(9CI)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Urinary tract infection [ICD-11: GC08; ICD-10: N39, N39.0; ICD-9: 599] | Approved | [1] | |
| Therapeutic Class |
Antiinfective Agents
|
|||
| Structure |
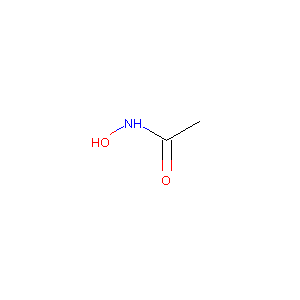 |
Download2D MOL |
||
| Formula |
C2H5NO2
|
|||
| Canonical SMILES |
CC(=O)NO
|
|||
| InChI |
1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4)
|
|||
| InChIKey |
RRUDCFGSUDOHDG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 546-88-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9027, 71235, 445755, 585483, 585486, 838108, 3136864, 4560833, 7847287, 7888061, 8144458, 8149186, 8151369, 10321139, 10526548, 11335996, 11361235, 11362774, 11365336, 11367898, 11371390, 11373695, 11376060, 11462207, 11483834, 11487925, 11490101, 11491953, 11493834, 12498511, 15218626, 17435944, 22395295, 24845057, 24849786, 26611590, 26679199, 26702620, 26705705, 26711850, 26736917, 26736932, 26747835, 26747836, 29221178, 30983098, 31559552, 39222143, 46508546, 47515358
|
|||
| ChEBI ID |
CHEBI:27777
|
|||
| ADReCS Drug ID | BADD_D00028 | |||
| SuperDrug ATC ID |
G04BX03
|
|||
| SuperDrug CAS ID |
cas=000546883
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Urease (Bact ureC) | Target Info | Inhibitor | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 018749. | |||
| REF 2 | Enzymatic, immunological and phylogenetic characterization of Brucella suis urease. BMC Microbiol. 2008 Jul 19;8:121. | |||
| REF 3 | Amines and oximes derived from deoxybenzoins as Helicobacter pylori urease inhibitors. Eur J Med Chem. 2009 May;44(5):2246-51. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

