Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R1VR
|
|||
| Drug Name |
Fluphenazine
|
|||
| Synonyms |
FLUPHENAZINE; Triflumethazine; Fluorophenazine; Fluorphenazine; Fluorfenazine; Siqualon; Elinol; 69-23-8; Phthorphenazine; Vespazine; Ftorphenazine; Siqualine; Sevinol; Pacinol; Fluphenazinum; Flufenazina; Prolixin; Flufenazin; Dapotum; Flufenazina [DCIT]; Yespazine; SQ 4918; Fluphenazine [INN:BAN]; Moditen (Tabl or elixir); Fluphenazinum [INN-Latin]; 2-(4-(3-(2-(trifluoromethyl)-10H-phenothiazin-10-yl)propyl)piperazin-1-yl)ethanol; 10-(3-(2-Hydroxyethyl)piperazinopropyl)-2-(trifluoromethyl)phenothiazine; UNII-S79426A41Z; HSDB 3334; Dapotum; Prolixine; Fluphenazine hydrochloride; Moditen Hcl; Permitil Concentrate; Prolixin Concentrate; S94; Anatensol (TN); Apo-Fluphenazine; Dapotum (TN); Dapotum D (TN); Dapotum Injektion (TN); Decanoate (TN); Deconoate (TN); Enanthate (TN); Fludecate (TN); Flunanthate (TN); Fluphenazine (INN); Hydrochloride, Fluphenazine; Lyogen (TN); Modecate (TN); Moditen (TN); Moditen Enanthate Injection (TN); Omca (TN); Permitil (TN); Prolixin (TN); Sediten (TN); Selecten (TN); Sevinol (TN); Sinqualone (TN); Trancin (TN)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Middle East Respiratory Syndrome (MERS) [ICD-11: 1D64] | Investigative | [1] | |
| Severe acute respiratory syndrome (SARS) [ICD-11: 1D65] | Investigative | [1], [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Bristol-Myers Squibb
|
|||
| Structure |
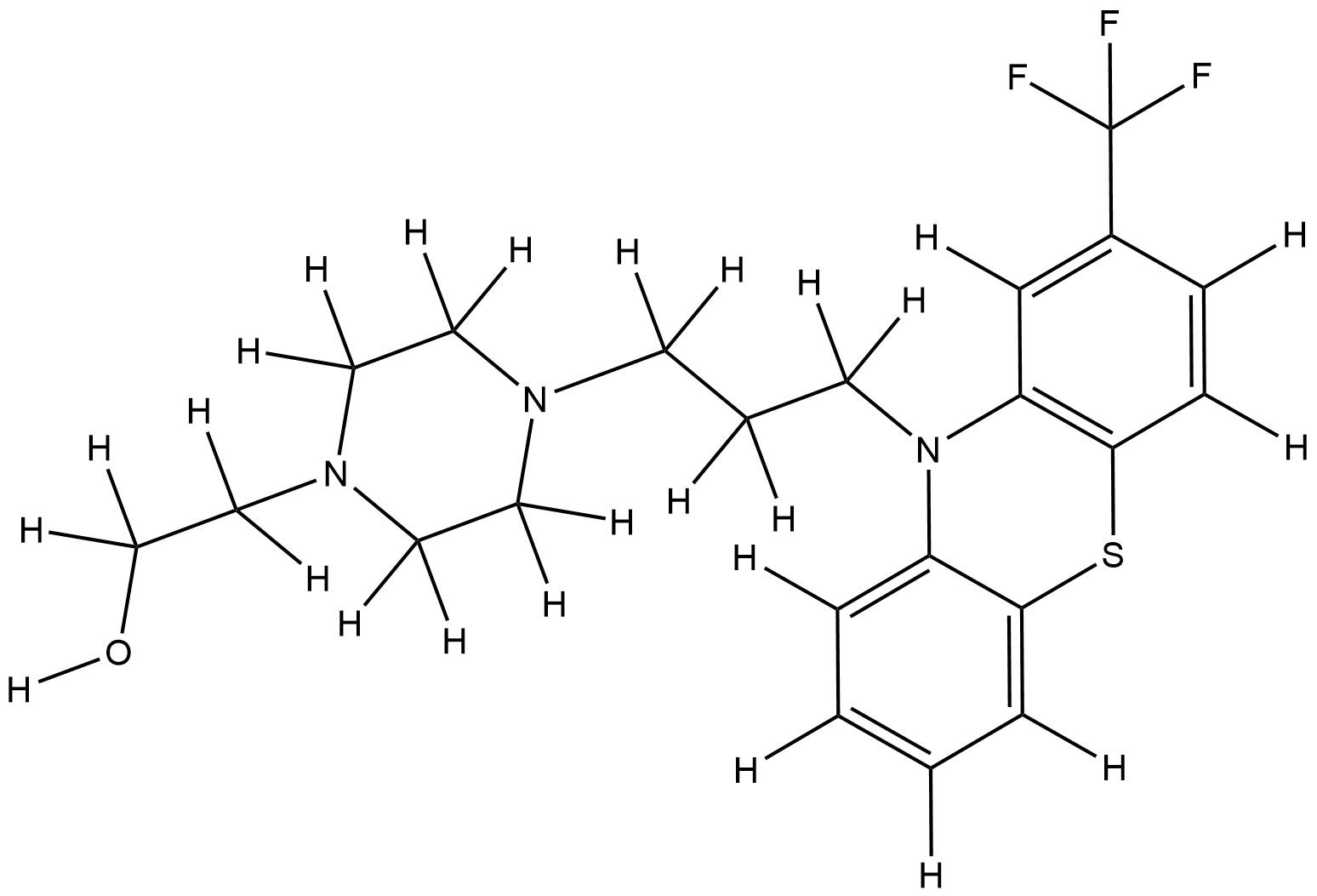 |
Download2D MOL |
||
| Formula |
C22H26F3N3OS
|
|||
| Canonical SMILES |
C1CN(CCN1CCCN2C3=CC=CC=C3SC4=C2C=C(C=C4)C(F)(F)F)CCO
|
|||
| InChI |
1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2
|
|||
| InChIKey |
PLDUPXSUYLZYBN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 69-23-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9223, 606306, 841998, 5329924, 7979286, 8152146, 10532904, 11111179, 11111180, 11212325, 11335686, 11360925, 11363183, 11364798, 11365745, 11367360, 11368307, 11369922, 11372419, 11372962, 11374514, 11375522, 11376469, 11378089, 11461897, 11466348, 11467468, 11485013, 11486275, 11489015, 11491235, 11492643, 11494103, 14832617, 24263032, 26751625, 29222507, 46506645, 47291093, 47291094, 47365145, 47588956, 47588957, 47662238, 47662239, 47736435, 47885368, 48110419, 48259193, 48259194
|
|||
| ChEBI ID |
CHEBI:5123
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN clathrin-mediated endocytosis (RME) | Target Info | Inhibitor | [1], [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Testing of Middle East respiratory syndrome coronavirus replication inhibitors for the ability to block viral entry. Antimicrob Agents Chemother. 2015 Jan;59(1):742-4. | |||
| REF 2 | Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014 Aug;58(8):4885-93. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

