Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q6MY
|
|||
| Drug Name |
Nafamostat
|
|||
| Synonyms |
FUT-175; Nafamostat [INN]; Nafamostatum [Latin]; UNII-Y25LQ0H97D; Nafamstat; Nafamstat Mesilate; 6-Amidino2-naphthyl 4-guanidinobenzoate; Y25LQ0H97D; CHEMBL273264; C19H17N5O2; Nafamostat (INN); p-Guanidinobenzoic acid ester with 6-hydroxy-2-naphthamidine; Nafamostatum
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 2/3 | [1] | |
| Middle East Respiratory Syndrome (MERS) [ICD-11: 1D64] | Preclinical | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
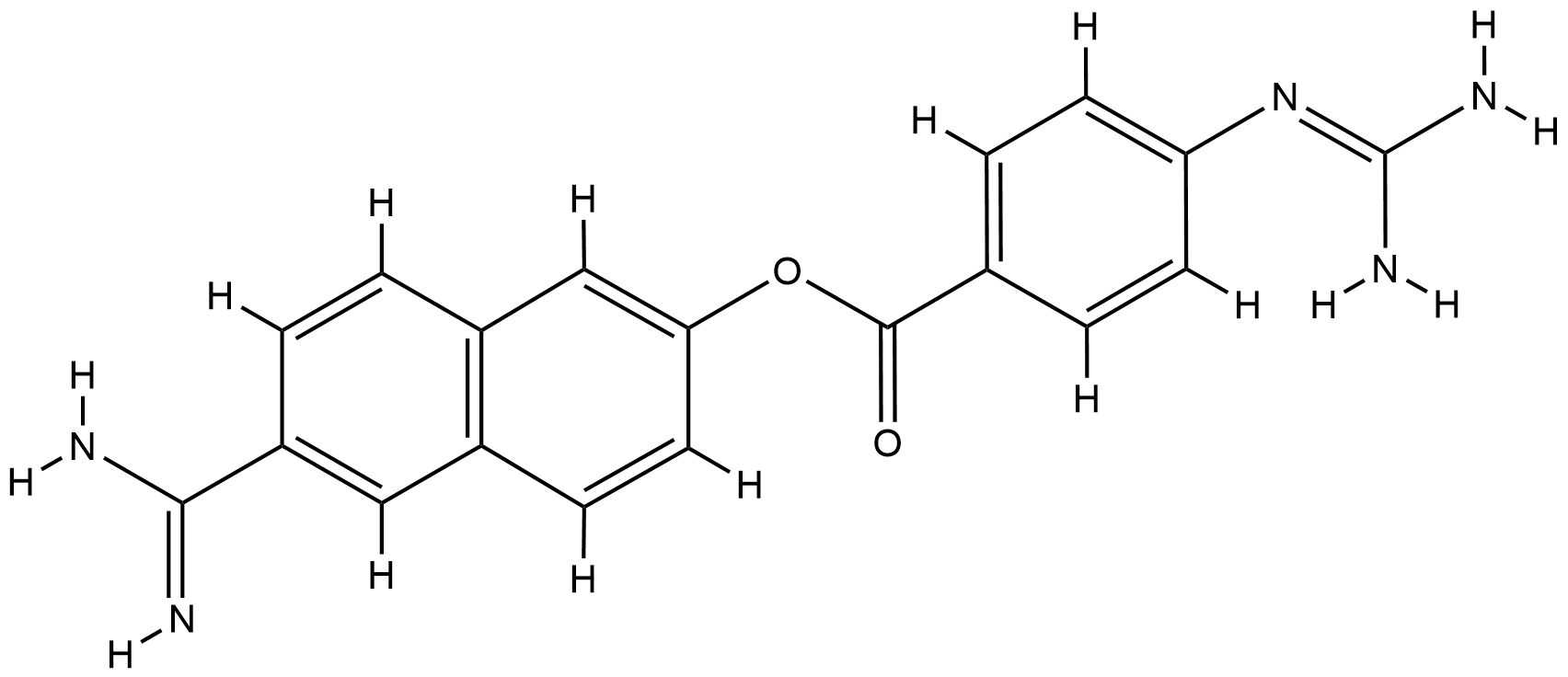 |
Download2D MOL |
||
| Formula |
C19H17N5O2
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C(=O)OC2=CC3=C(C=C2)C=C(C=C3)C(=N)N)N=C(N)N
|
|||
| InChI |
1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24)
|
|||
| InChIKey |
MQQNFDZXWVTQEH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 81525-10-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5096568, 14778337, 26754619, 29216079, 29223510, 47365353, 50065252, 50110097, 50334592, 57322261, 89650190, 91615064, 96024928, 103170416, 103824925, 103958902, 104306334, 117575270, 124892269, 125823849, 128633897, 129980079, 131327125, 134222539, 134339097, 134340258, 135062511, 137051650, 137120697, 138817266, 142058227, 164230816, 164813659, 178101079, 179151201, 184545971, 186009066, 198971060, 210279319, 210281642, 212296217, 223365680, 223401665, 226503299, 251916771, 251918010, 252480071
|
|||
| ChEBI ID |
CHEBI:135466
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | COVID-19 spike glycoprotein (S) | Target Info | Inhibitor | [3] |
| MERS-CoV spike glycoprotein (S) | Target Info | Inhibitor | [2] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04352400) Efficacy of Nafamostat in Covid-19 Patients (RACONA Study). U.S. National Institutes of Health. | |||
| REF 2 | Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay Antimicrob Agents Chemother. 2016 Oct 21;60(11):6532-6539. | |||
| REF 3 | Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020 Mar;19(3):149-150. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

