Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q5UQ
|
|||
| Former ID |
DNCL003653
|
|||
| Drug Name |
Exjade
|
|||
| Synonyms |
Deferasirox
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Iron overload disease [ICD-11: 5C64.10; ICD-10: E83.1, G23.0] | Approved | [1] | |
| Company |
Novartis
|
|||
| Structure |
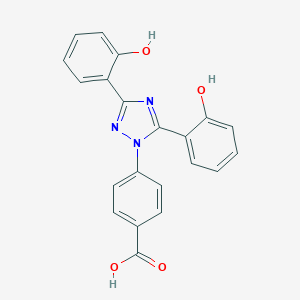 |
Download2D MOL |
||
| Formula |
C21H15N3O4
|
|||
| Canonical SMILES |
C1=CC=C(C(=C1)C2=NN(C(=N2)C3=CC=CC=C3O)C4=CC=C(C=C4)C(=O)O)O
|
|||
| InChI |
1S/C21H15N3O4/c25-17-7-3-1-5-15(17)19-22-20(16-6-2-4-8-18(16)26)24(23-19)14-11-9-13(10-12-14)21(27)28/h1-12,25-26H,(H,27,28)
|
|||
| InChIKey |
BOFQWVMAQOTZIW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 201530-41-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14853198, 17397765, 29157338, 39475182, 46506791, 49693590, 50070193, 50071321, 50362477, 53787326, 57364789, 104179183, 104253329, 114000977, 124757448, 124772227, 125164252, 126592965, 126666897, 135129477, 137008102, 137248684, 143497484, 144115893, 144206479, 151995258, 152236475, 160964846, 162188004, 170464723, 175266885, 179117014, 184545799, 186022468, 223384180, 223663416, 226443599, 241050655, 251892008, 252219959, 252390148, 252471362
|
|||
| ChEBI ID |
CHEBI:49005
|
|||
| ADReCS Drug ID | BADD_D00594 | |||
| SuperDrug ATC ID |
V03AC03
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Iron (Fe) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2005 approvals: Safety first. Nature Reviews Drug Discovery 5, 92-93 (February 2006). | |||
| REF 2 | Deferasirox : a review of its use in the management of transfusional chronic iron overload. Drugs. 2007;67(15):2211-30. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

