Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q3ES
|
|||
| Former ID |
DCL000051
|
|||
| Drug Name |
APD668
|
|||
| Synonyms |
832714-46-2; APD-668; CHEMBL1775179; Isopropyl 4-((1-(2-fluoro-4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)oxy)piperidine-1-carboxylate; isopropyl 4-(1-(2-fluoro-4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate; 1-Piperidinecarboxylic acid,4-[[1-[2-fluoro-4-(methylsulfonyl)phenyl]-1H-pyrazolo[3,4-d]pyrimidin-4-yl]oxy]-, 1-methylethyl ester; SCHEMBL389323; AOB6271; XTRUQJBVQBUKSQ-UHFFFAOYSA-N; MolPort-039-331-631; EX-A1795; BCP10207; BDBM50343442; ZINC68266967
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Discontinued in Phase 1 | [1] | |
| Company |
Arena Pharma.
|
|||
| Structure |
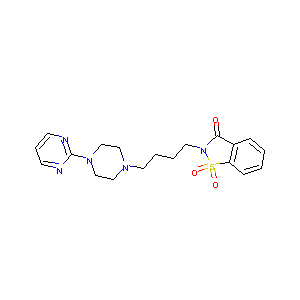 |
Download2D MOL |
||
| Formula |
C21H24FN5O5S
|
|||
| Canonical SMILES |
CC(C)OC(=O)N1CCC(CC1)OC2=NC=NC3=C2C=NN3C4=C(C=C(C=C4)S(=O)(=O)C)F
|
|||
| InChI |
1S/C21H24FN5O5S/c1-13(2)31-21(28)26-8-6-14(7-9-26)32-20-16-11-25-27(19(16)23-12-24-20)18-5-4-15(10-17(18)22)33(3,29)30/h4-5,10-14H,6-9H2,1-3H3
|
|||
| InChIKey |
XTRUQJBVQBUKSQ-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7603533, 8184984, 11114236, 12013978, 14903693, 43114717, 47285840, 47731233, 49883567, 50326777, 57313863, 85209405, 85788167, 91746214, 103166960, 103918673, 104311449, 104827256, 117598635, 119525529, 129335952, 134338757, 134340130, 135015567, 135650411, 135698194, 137231966, 142141651, 144204513, 162023316, 162222165, 163135249, 170466380, 175611931, 179151468, 184536638, 198972236, 202544113, 224910665, 226458694, 252156151, 252330661, 252450956, 252562161
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucose-dependent insulinotropic receptor (GPR119) | Target Info | Agonist | [2] |
| KEGG Pathway | cAMP signaling pathway | |||
| Insulin secretion | ||||
| WikiPathways | Incretin Synthesis, Secretion, and Inactivation | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023882) | |||
| REF 2 | Announces Initiation of Phase 1 Clinical Trial of Arena Type 2 Diabetes Drug Candidate in Collaboration With Ortho-McNeil. Arena Pharmaceuticals, Inc. FEBRUARY 07, 2006. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

