Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0PU8T
|
|||
| Former ID |
DNCL001872
|
|||
| Drug Name |
E5555
|
|||
| Synonyms |
Atopaxar
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute coronary syndrome [ICD-11: BA41; ICD-9: 411.1] | Phase 2 | [1], [2] | |
| Company |
Eisai
|
|||
| Structure |
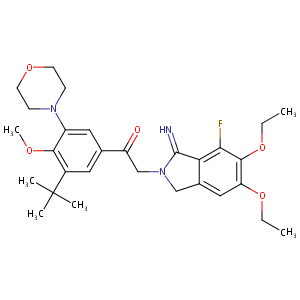 |
Download2D MOL |
||
| Formula |
C29H38FN3O5
|
|||
| Canonical SMILES |
CCOC1=C(C(=C2C(=C1)CN(C2=N)CC(=O)C3=CC(=C(C(=C3)N4CCOCC4)OC)C(C)(C)C)F)OCC
|
|||
| InChI |
1S/C29H38FN3O5/c1-7-37-23-15-19-16-33(28(31)24(19)25(30)27(23)38-8-2)17-22(34)18-13-20(29(3,4)5)26(35-6)21(14-18)32-9-11-36-12-10-32/h13-15,31H,7-12,16-17H2,1-6H3
|
|||
| InChIKey |
QWKAUGRRIXBIPO-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 751475-53-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4048). | |||
| REF 2 | ClinicalTrials.gov (NCT00619164) A Double-Blind Study of E5555 in Japanese Patients With Acute Coronary Syndrome. U.S. National Institutes of Health. | |||
| REF 3 | Safety and tolerability of atopaxar in the treatment of patients with acute coronary syndromes: the lessons from antagonizing the cellular effects of Thrombin-Acute Coronary Syndromes Trial. Circulation. 2011 May 3;123(17):1843-53. | |||
| REF 4 | Atopaxar and its effects on markers of platelet activation and inflammation: results from the LANCELOT CAD program. J Thromb Thrombolysis. 2012 Jul;34(1):36-43. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

