Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P5GE
|
|||
| Former ID |
DAP000507
|
|||
| Drug Name |
Dibucaine
|
|||
| Synonyms |
Cincainum; Cinchocaine; Cinchocainum; Cincocaina; Cincocainio; Dermacaine; Dibucain; Dibucainum; Nupercainal; Nupercaine; Percamine; Sovcaine; Cinchocaine HCL; Cinchocaine hydrochloride; Cincocaina [DCIT]; Dibucaine Base; Dibucaine [USP]; Alpha-Butyloxycinchoninic acid diethylethylenediamide; Cincain (TN); Cinchocaine (INN);Cinchocainum [INN-Latin]; Cincocainio [INN-Spanish]; Dibucaine (USP); Nupercainal (TN); Nupercainal (VAN); Nupercaine (TN); Sovcaine (TN); Alpha-Butyloxycinchonic acid-gamma-diethylethylenediamine; N-[2-(Diethylamino)ethyl]-2-butoxycinchoninamide; N-(2-(Diethylamino)ethyl)-2-butoxycinchoninamide; QUINOLINE,2-BUTOXY,4-CARBOXY,(N-TRIETHYLAMINO) AMIDE CINCHOCAIN; 2-Butoxy-N-(2-(diethylamino)ethyl)cinchoninamide; 2-Butoxy-N-(beta-diethylaminoethyl)cinchoninamide; 2-Butoxy-N-(beta.-diethylaminoethyl)cinchoninamide; 2-Butoxy-N-[2-(diethylamino)ethyl]-4-quinolinecarboxamide; 2-Butoxy-N-[2-(diethylamino)ethyl]cinchoninamide; 2-Butoxy-quinoline-4-carboxylic acid (2-diethylamino-ethyl)-amide; 2-Butoxyquinoline-4-carboxylic acid diethylaminoethylamide; 2-N-Butoxy-N-(2-diethylaminoethyl)cinchoninamide; 2-butoxy-N-(2-diethylaminoethyl)quinoline-4-carboxamide; 2-butoxy-N-(alpha-diethylaminoethyl)cinchoninamide; 2-butoxy-N-[2-(diethylamino)ethyl]quinoline-4-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anaesthesia [ICD-11: 9A78.6; ICD-9: 338] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Anesthetics
|
|||
| Company |
Novartis Pharmaceuticals Corp
|
|||
| Structure |
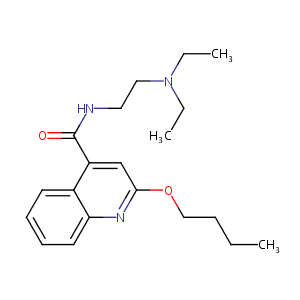 |
Download2D MOL |
||
| Formula |
C20H29N3O2
|
|||
| Canonical SMILES |
CCCCOC1=NC2=CC=CC=C2C(=C1)C(=O)NCCN(CC)CC
|
|||
| InChI |
1S/C20H29N3O2/c1-4-7-14-25-19-15-17(16-10-8-9-11-18(16)22-19)20(24)21-12-13-23(5-2)6-3/h8-11,15H,4-7,12-14H2,1-3H3,(H,21,24)
|
|||
| InChIKey |
PUFQVTATUTYEAL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 85-79-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10081, 436088, 588150, 855529, 5385819, 7847798, 7978947, 8151924, 10321264, 10537030, 10589300, 11112228, 11335507, 11360746, 11363822, 11366384, 11368946, 11371487, 11374188, 11377108, 11461718, 11466104, 11467224, 11484857, 11485948, 11488817, 11490200, 11492320, 11494742, 14802538, 24893238, 29222172, 46506734, 47440166, 47515231, 47515232, 47662196, 47662197, 47736388, 47810660, 49698853, 49903695, 50100394, 50100395, 50455743, 56424123, 56436944, 57321562, 57654061, 85174672
|
|||
| ChEBI ID |
CHEBI:247956
|
|||
| ADReCS Drug ID | BADD_D00651 | |||
| SuperDrug ATC ID |
C05AD04; D04AB02; N01BB06; S01HA06; S02DA04
|
|||
| SuperDrug CAS ID |
cas=000085790
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated sodium channel alpha Nav1.5 (SCN5A) | Target Info | Blocker | [4] |
| KEGG Pathway | Adrenergic signaling in cardiomyocytes | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Cardiac Progenitor Differentiation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7159). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 006203. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Halothane attenuates the cerebroprotective action of several Na+ and Ca2+ channel blockers via reversal of their ion channel blockade. Eur J Pharmacol. 2002 Oct 4;452(2):175-81. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

