Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P1UX
|
|||
| Former ID |
DAP000054
|
|||
| Drug Name |
Venlafaxine
|
|||
| Synonyms |
Efectin; Elafax; Venlafaxina; VenlafaxineXR; Venlafaxinum; Venlafaxine ER; Wy 45030; Efectin (TN); Venlafaxina [INN-Spanish];Venlafaxine (Effexor); Venlafaxine (INN); Venlafaxine [BAN:INN]; Venlafaxine [INN:BAN]; Venlafaxinum [INN-Latin]; N,N-dimethyl-2-(1-hydroxycyclohexyl)-2-(4-methoxyphenyl)ethylamine; 1-[(1R)-2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol; 1-[2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol; 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexan-1-ol; 1-[2-dimethylamino-1-(4-methoxyphenyl)ethyl]cyclohexan-1-ol; 1-{2-(dimethylamino)-1-[4-(methyloxy)phenyl]ethyl}cyclohexanol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Depression [ICD-11: 6A70-6A7Z; ICD-9: 311] | Approved | [1], [2] | |
| Therapeutic Class |
Antidepressants
|
|||
| Company |
Wyeth
|
|||
| Structure |
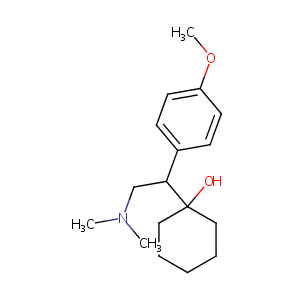 |
Download2D MOL |
||
| Formula |
C17H27NO2
|
|||
| Canonical SMILES |
CN(C)CC(C1=CC=C(C=C1)OC)C2(CCCCC2)O
|
|||
| InChI |
1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3
|
|||
| InChIKey |
PNVNVHUZROJLTJ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 93413-69-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9396, 5430653, 7980876, 8153474, 11342113, 11362296, 11364565, 11367127, 11369689, 11371976, 11374728, 11377851, 11484319, 11487698, 11488358, 11490804, 11492929, 11495485, 15222119, 17425507, 26612651, 26679955, 26748923, 26748924, 29224693, 46504593, 47583253, 48029197, 48416696, 49958155, 50107485, 51004905, 57288870, 57322883, 85787821, 85789257, 90341232, 92124669, 92307842, 92309100, 92714246, 94568830, 96025353, 103188954, 103923614, 104309869, 117865170, 118043482, 124637412, 124891843
|
|||
| ChEBI ID |
CHEBI:9943
|
|||
| ADReCS Drug ID | BADD_D02346 ; BADD_D02347 | |||
| SuperDrug ATC ID |
N06AX16
|
|||
| SuperDrug CAS ID |
cas=093413695
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Norepinephrine transporter (NET) | Target Info | Inhibitor | [3] |
| Serotonin transporter (SERT) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Serotonergic synapse | |||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | 5HT1 type receptor mediated signaling pathway | |||
| 5HT2 type receptor mediated signaling pathway | ||||
| 5HT3 type receptor mediated signaling pathway | ||||
| 5HT4 type receptor mediated signaling pathway | ||||
| Adrenaline and noradrenaline biosynthesis | ||||
| Reactome | Na+/Cl- dependent neurotransmitter transporters | |||
| WikiPathways | Monoamine Transport | |||
| SIDS Susceptibility Pathways | ||||
| NRF2 pathway | ||||
| Synaptic Vesicle Pathway | ||||
| Serotonin Transporter Activity | ||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7321). | |||
| REF 2 | Desvenlafaxine in the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2009;5:127-36. | |||
| REF 3 | Clinically relevant drug interactions with new generation antidepressants and antipsychotics. Ther Umsch. 2009 Jun;66(6):485-92. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

