Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0OL6O
|
|||
| Former ID |
DAP001024
|
|||
| Drug Name |
Methyl aminolevulinate
|
|||
| Synonyms |
Metvix; Aminolevulinic acid methyl ester; Methyl 5-aminolevulinate; Methyl delta-aminolevulinate; Methyl5-amino-4-oxopentanoate; Levulinic acid, 5-amino-, methyl ester; Pentanoic acid, 5-amino-4-oxo-, methyl ester; 5-Aminolevulinic acid methyl ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acne vulgaris [ICD-11: ED80; ICD-10: L70.0; ICD-9: 706.1] | Approved | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
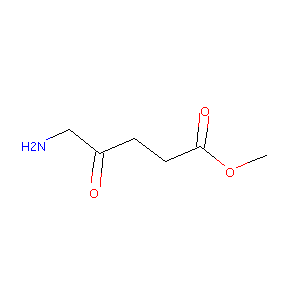 |
Download2D MOL |
||
| Formula |
C6H11NO3
|
|||
| Canonical SMILES |
COC(=O)CCC(=O)CN
|
|||
| InChI |
1S/C6H11NO3/c1-10-6(9)3-2-5(8)4-7/h2-4,7H2,1H3
|
|||
| InChIKey |
YUUAYBAIHCDHHD-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 33320-16-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3152707, 6729582, 10253237, 14747821, 46233440, 46507004, 50070563, 50071305, 50100968, 75370426, 96024892, 99333768, 103749693, 104171381, 113438636, 124883561, 124883562, 125824630, 126570614, 129851228, 132503116, 134221978, 134224946, 134338292, 135141172, 137127158, 142505868, 160964329, 165372994, 175268075, 179151184, 203088301, 223541399, 223817042, 225144187, 226399702
|
|||
| ChEBI ID |
CHEBI:724125
|
|||
| SuperDrug ATC ID |
L01XD03
|
|||
| SuperDrug CAS ID |
cas=079416276
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Ferrochelatase (FECH) | Target Info | Activator | [3] |
| BioCyc | Heme biosynthesis | |||
| Heme biosynthesis from uroporphyrinogen-III I | ||||
| KEGG Pathway | Porphyrin and chlorophyll metabolism | |||
| Metabolic pathways | ||||
| Panther Pathway | Heme biosynthesis | |||
| Pathwhiz Pathway | Porphyrin Metabolism | |||
| Pathway Interaction Database | HIF-1-alpha transcription factor network | |||
| WikiPathways | Heme Biosynthesis | |||
| Metabolism of porphyrins | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021415. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res. 2009 May 15;15(10):3333-43. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

