Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0OC4O
|
|||
| Former ID |
DNCL002462
|
|||
| Drug Name |
RG7388
|
|||
| Synonyms |
Idasanutlin; RG7388; 1229705-06-9; Idasanutlin (RG-7388); RG-7388; UNII-QSQ883V35U; QSQ883V35U; CHEMBL2402737; Benzoic acid, 4-((((2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethylpropyl)-2-pyrrolidinyl)carbonyl)amino)-3-methoxy-; RO5503781; Idasanutlin [USAN:INN]; Benzoic acid, 4-[[[(2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethylpropyl)-2-pyrrolidinyl]carbonyl]amino]-3-methoxy-; RG-7388;Idasanutlin; RO-5503781; SCHEMBL442856
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60; ICD-9: 205] | Phase 3 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 3 | [2] | ||
| Polycythemia vera [ICD-11: 2A20.4; ICD-10: D45; ICD-9: 238.4] | Phase 2 | [3] | ||
| Haematological malignancy [ICD-11: 2B33.Y] | Phase 1 | [4] | ||
| Company |
Roche
|
|||
| Structure |
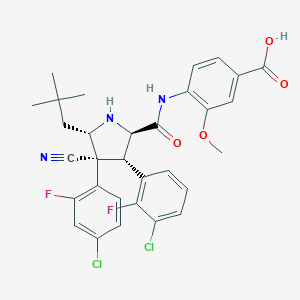 |
Download2D MOL |
||
| Formula |
C31H29Cl2F2N3O4
|
|||
| Canonical SMILES |
CC(C)(C)CC1C(C(C(N1)C(=O)NC2=C(C=C(C=C2)C(=O)O)OC)C3=C(C(=CC=C3)Cl)F)(C#N)C4=C(C=C(C=C4)Cl)F
|
|||
| InChI |
1S/C31H29Cl2F2N3O4/c1-30(2,3)14-24-31(15-36,19-10-9-17(32)13-21(19)34)25(18-6-5-7-20(33)26(18)35)27(38-24)28(39)37-22-11-8-16(29(40)41)12-23(22)42-4/h5-13,24-25,27,38H,14H2,1-4H3,(H,37,39)(H,40,41)/t24-,25-,27+,31-/m0/s1
|
|||
| InChIKey |
TVTXCJFHQKSQQM-LJQIRTBHSA-N
|
|||
| CAS Number |
CAS 1229705-06-9
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02545283) A Study of Idasanutlin With Cytarabine Versus Cytarabine Plus Placebo in Participants With Relapsed or Refractory Acute Myeloid Leukemia (AML) (MIRROS). U.S. National Institutes of Health. | |||
| REF 2 | Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | ClinicalTrials.gov (NCT02407080) Open Label Study of Single Agent Oral RG7388 in Patients With Polycythemia Vera and Essential Thrombocythemia. U.S. National Institutes of Health. | |||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1799). | |||
| REF 6 | Pre-clinical evaluation of the MDM2-p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma. Oncotarget. 2015 Apr 30;6(12):10207-21. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

