Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O4NR
|
|||
| Former ID |
DIB001923
|
|||
| Drug Name |
LTB4
|
|||
| Synonyms |
Leukotriene B4; LEUKOTRIENE B4; 5,12-Dihete; 71160-24-2; 5,12-Hete; UNII-1HGW4DR56D; (5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,14-tetraenoic acid; 1HGW4DR56D; CHEMBL65061; 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid; CHEBI:15647; VNYSSYRCGWBHLG-AMOLWHMGSA-N; [5S,12R]-Dihydroxy-[6Z,8E,10E,14Z]-eicosatetraenoic acid; Leukotriene B; 5(S),12(R)-Dihydroxy-6-cis-8-trans-10-trans-14-cis-eicosatetraenoic Acid; (S-(R*,S*-(E,Z,E,Z)))-5,12-Dihydroxy-6,8,10,14-eicosatetraenoic acid; 6,8,10,14-Eicosatetraenoic acid,; Leukotriene B4 agonist (HIV infection), LTB4 Sweden AB; LEUKOTRIENE_B4
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Phase 2 | [1] | |
| Company |
LTB4 Sweden AB
|
|||
| Structure |
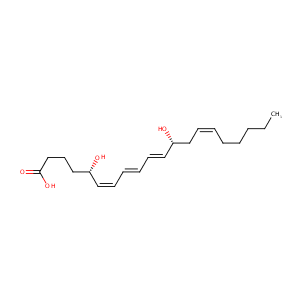 |
Download2D MOL |
||
| Formula |
C20H32O4
|
|||
| Canonical SMILES |
CCCCCC=CCC(C=CC=CC=CC(CCCC(=O)O)O)O
|
|||
| InChI |
1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1
|
|||
| InChIKey |
VNYSSYRCGWBHLG-AMOLWHMGSA-N
|
|||
| CAS Number |
CAS 71160-24-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5240, 801682, 4266069, 7979751, 8143279, 8616293, 14753488, 15316444, 24896256, 26754791, 26754792, 26754793, 26759044, 39289598, 47217017, 47515561, 47811004, 48110702, 48259480, 48334747, 49964833, 50110780, 53790345, 53801088, 57357761, 79593894, 92126167, 92309780, 99300694, 99302212, 103256813, 103925923, 104046421, 113853357, 126523796, 127316819, 127316820, 127316821, 127316822, 127316823, 127316824, 127316825, 127316826, 127316827, 127316828, 127316829, 127316830, 127316831, 134341607, 135010128
|
|||
| ChEBI ID |
CHEBI:15647
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Leukotriene B4 receptor 1 (LTB4R) | Target Info | Agonist | [2], [3] |
| Leukotriene B4 receptor 2 (LTB4R2) | Target Info | Inhibitor | [4] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Calcium signaling pathway | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Panther Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | |||
| Reactome | Leukotriene receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Nucleotide GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Spinal Cord Injury | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00251537) A Pilot Study of LTB4 in HIV-1 Infected Adults in LTB4 Sweden AB. | |||
| REF 2 | LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015 Mar;21(3):239-47. | |||
| REF 3 | Synthesis and pharmacological activity of SC-53228, a leukotriene B4 receptor antagonist with high intrinsic potency and selectivity, Bioorg. Med. Chem. Lett. 4(6):811-816 (1994). | |||
| REF 4 | Diaryl ether/carboxylic acid derivatives of LY255283: Receptor antagonists of leukotriene B4, Bioorg. Med. Chem. Lett. 3(10):1985-1990 (1993). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

