Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N5JZ
|
|||
| Former ID |
DNCL002403
|
|||
| Drug Name |
PF-04691502
|
|||
| Synonyms |
PF-04691502; 1013101-36-4; PF 04691502; UNII-4W39NS61KI; 4W39NS61KI; 2-amino-8-((1r,4r)-4-(2-hydroxyethoxy)cyclohexyl)-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7(8H)-one; CHEMBL1234354; PF04691502; 2-Amino-8-[trans-4-(2-Hydroxyethoxy)cyclohexyl]-6-(6-Methoxypyridin-3-Yl)-4-Methylpyrido[2,3-D]pyrimidin-7(8h)-One; 2-Amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Endometrial cancer [ICD-11: 2C76; ICD-9: 182] | Phase 2 | [1], [2] | |
| Company |
Pfizer New York, NY
|
|||
| Structure |
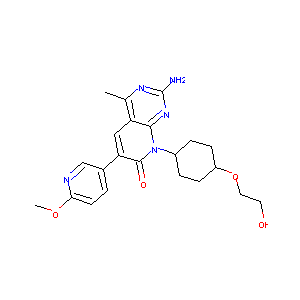 |
Download2D MOL |
||
| Formula |
C22H27N5O4
|
|||
| Canonical SMILES |
CC1=C2C=C(C(=O)N(C2=NC(=N1)N)C3CCC(CC3)OCCO)C4=CN=C(C=C4)OC
|
|||
| InChI |
1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)
|
|||
| InChIKey |
XDLYKKIQACFMJG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1013101-36-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
56384319, 92727817, 93578219, 104250512, 131549272, 136340126, 136349582, 136367713, 136946656, 140658184, 140658275, 143499835, 152233129, 152258633, 160647468, 160859396, 162011694, 162038145, 162169454, 162202644, 164041993, 174006461, 176223198, 185997022, 189561524, 196680010, 198978074, 223377891, 223705055, 224173302, 227598568, 227598573, 228144234, 235430003, 238964975, 243347842, 248313923, 249565619, 251963203, 252110191, 252158386, 252160513, 252216040, 252451776
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7936). | |||
| REF 2 | ClinicalTrials.gov (NCT01430585) Pre-Operative Study of PF-4691502 With Letrozole Compared To Letrozole Alone In Patients With Early Breast Cancer. U.S. National Institutes of Health. | |||
| REF 3 | PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity.Mol Cancer Ther.2011 Nov;10(11):2189-99. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

