Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N1EW
|
|||
| Former ID |
DIB003843
|
|||
| Drug Name |
DuP-532
|
|||
| Synonyms |
Dup-532; Dup 532; 124750-95-4; CHEMBL443269; 1H-Imidazole-5-carboxylicacid,4-(1,1,2,2,2-pentafluoroethyl)-2-propyl-1-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-; dup532; ACMC-20mr6i; SCHEMBL62; AC1L1TVZ; AC1Q4ICA; CTK4B4065; BDBM82428; DTXSID00154476; PDSP2_000123; PDSP1_000124; PDSP1_000123; BDBM50230908; 2-Propyl-4-pentafluoroethyl-1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)imidazole-5-carboxylic acid; CAS_124750-95-4; CB91356279; L002873; 1H-Imidazole-5-carboxylic acid, 4-(pentafluoroethyl)-2-propyl-1-((2'-(1H-te
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Discontinued in Phase 1 | [1] | |
| Company |
Bristol-Myers Squibb Pharma Co
|
|||
| Structure |
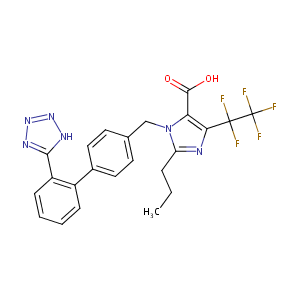 |
Download2D MOL |
||
| Formula |
C23H19F5N6O2
|
|||
| Canonical SMILES |
CCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NNN=N4)C(=O)O)C(C(F)(F)F)(F)F
|
|||
| InChI |
1S/C23H19F5N6O2/c1-2-5-17-29-19(22(24,25)23(26,27)28)18(21(35)36)34(17)12-13-8-10-14(11-9-13)15-6-3-4-7-16(15)20-30-32-33-31-20/h3-4,6-11H,2,5,12H2,1H3,(H,35,36)(H,30,31,32,33)
|
|||
| InChIKey |
RQGDXPDTZWGCQI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 124750-95-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Angiotensin II receptor (AGTR) | Target Info | Modulator | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Vascular smooth muscle contraction | ||||
| Renin-angiotensin system | ||||
| Renin secretion | ||||
| Pathways in cancer | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001160) | |||
| REF 2 | DuP 532, an angiotensin II receptor antagonist: first administration and comparison with losartan. Clin Pharmacol Ther. 1997 Jan;61(1):59-69. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

