Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N0RN
|
|||
| Former ID |
DNCL003486
|
|||
| Drug Name |
Marizomib
|
|||
| Synonyms |
MARIZOMIB; salinosporamide A; 437742-34-2; (-)-Salinosporamide A; UNII-703P9YDP7F; NPI-0052; NPI 0052; ML 858; 703P9YDP7F; CHEBI:48045; (1R,4R,5S)-4-(2-chloroethyl)-1-[(S)-(1S)-cyclohex-2-en-1-yl(hydroxy)methyl]-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione; Marizomib [USAN:INN]; marizomibum; Marizomib (USAN/INN); Salinosporamide A (NPI-0052, Marizomib); SCHEMBL151667; CHEMBL371405; NGWSFRIPKNWYAO-SHTIJGAHSA-N; ZINC3990364; BDBM50398608; 2531AH; AKOS027323566; DB11762; Z-3093; D09640; 855517-10-1
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Glioblastoma of brain [ICD-11: 2A00.00; ICD-10: C71] | Phase 3 | [1] | |
| Malignant glioma [ICD-11: 2A00.0; ICD-9: 191] | Phase 1 | [2] | ||
| Multiple myeloma [ICD-11: 2A83; ICD-10: C90.0] | Phase 1 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [3] | ||
| Company |
Nereus Pharmaceuticals
|
|||
| Structure |
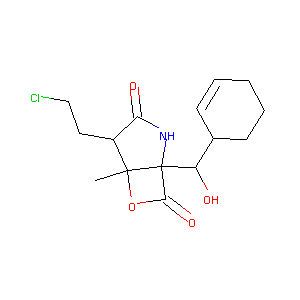 |
Download2D MOL |
||
| Formula |
C15H20ClNO4
|
|||
| Canonical SMILES |
CC12C(C(=O)NC1(C(=O)O2)C(C3CCCC=C3)O)CCCl
|
|||
| InChI |
1S/C15H20ClNO4/c1-14-10(7-8-16)12(19)17-15(14,13(20)21-14)11(18)9-5-3-2-4-6-9/h3,5,9-11,18H,2,4,6-8H2,1H3,(H,17,19)/t9-,10+,11+,14+,15+/m1/s1
|
|||
| InChIKey |
NGWSFRIPKNWYAO-SHTIJGAHSA-N
|
|||
| CAS Number |
CAS 437742-34-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:48045
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Proteasome (PS) | Target Info | Inhibitor | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03345095) A Phase III Trial of With Marizomib in Patients With Newly Diagnosed Glioblastoma (MIRAGE). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of Triphase Accelerator . | |||
| REF 4 | Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011 Mar;11(3):254-84. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

