Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N0BI
|
|||
| Former ID |
DNC003909
|
|||
| Drug Name |
SIBRAFIBAN
|
|||
| Synonyms |
Sibrafiban; UNII-YUE443B0NF; Sibrafiban [USAN:INN:BAN]; YUE443B0NF; Ro 48-3657; 172927-65-0; Ro 48-3657/001; Ethyl (Z)-((1-(N-((p-hydroxyamidino)benzoyl)-L-alanyl)-4-piperidyl)oxy)acetate; Ro-48-3657; ((1-(2-((4-(amino(hydroxyimino)methyl)benzoyl)amino)-1-oxopropyl)-4-piperidinyl)oxy)acetic acid ethyl ester; Sibrafiban [INN]; Acetic acid, ((1-(2-((4-(amino(hydroxyimino)methyl)benzoyl)amino)-1-oxopropyl)-4-piperidinyl)oxy)-, ethyl ester, (S-(Z))-; Sibrafiban [USAN]; Sibrafiban [MART.]; Sibrafiban [WHO-DD]; Xubix
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Angina pectoris [ICD-11: BA40; ICD-9: 413] | Discontinued in Phase 3 | [1] | |
| Cardiovascular disease [ICD-11: BA00-BE2Z] | Discontinued in Phase 3 | [2] | ||
| Structure |
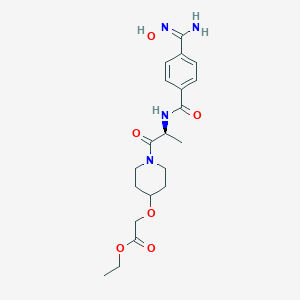 |
Download2D MOL |
||
| Formula |
C20H28N4O6
|
|||
| Canonical SMILES |
CCOC(=O)COC1CCN(CC1)C(=O)C(C)NC(=O)C2=CC=C(C=C2)C(=NO)N
|
|||
| InChI |
1S/C20H28N4O6/c1-3-29-17(25)12-30-16-8-10-24(11-9-16)20(27)13(2)22-19(26)15-6-4-14(5-7-15)18(21)23-28/h4-7,13,16,28H,3,8-12H2,1-2H3,(H2,21,23)(H,22,26)/t13-/m0/s1
|
|||
| InChIKey |
WBNUCLPUOSXSNJ-ZDUSSCGKSA-N
|
|||
| CAS Number |
CAS 170094-62-9
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006751) | |||
| REF 2 | Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50. | |||
| REF 3 | Pharmacokinetics and pharmacodynamics of sibrafiban, an orally administered GP IIb/IIIa antagonist, following coadministration of aspirin and heparin. J Clin Pharmacol. 2000 May;40(5):488-95. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

