Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0MW0N
|
|||
| Former ID |
DNCL002054
|
|||
| Drug Name |
Masitinib
|
|||
| Synonyms |
Masitinib; 790299-79-5; AB1010; Masatinib; Masitinib (AB1010); Masivet; AB-1010; AB 1010; UNII-M59NC4E26P; Masitinib [INN]; M59NC4E26P; 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-thiazolyl]amino]phenyl]benzamide; CHEMBL1908391; CHEBI:63450; Masitinib (INN); N-(4-Methyl-3-((4-(pyridin-3-yl)thiazol-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide; Q-201339; C28H30N6OS; N-(4-methyl-3-(4-(pyridin-3-yl)thiazol-2-ylamino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Amyotrophic lateral sclerosis [ICD-11: 8B60.0; ICD-9: 335.2] | Phase 3 | [1] | |
| Gastrointestinal stromal tumour [ICD-11: 2B5B] | Phase 3 | [1], [2] | ||
| Metastatic gastric or gastroesophageal junction cancer [ICD-11: 2D8Y; ICD-10: C15, C16] | Phase 3 | [1] | ||
| Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Phase 3 | [3] | ||
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 3 | [2] | ||
| Pancreatic cancer [ICD-11: 2C10] | Phase 3 | [2] | ||
| Company |
AB Science
|
|||
| Structure |
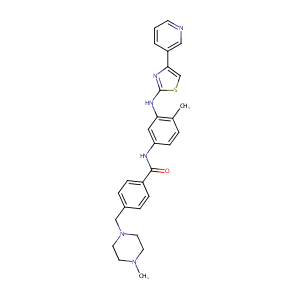 |
Download2D MOL |
||
| Formula |
C28H30N6OS
|
|||
| Canonical SMILES |
CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC(=CS4)C5=CN=CC=C5
|
|||
| InChI |
1S/C28H30N6OS/c1-20-5-10-24(16-25(20)31-28-32-26(19-36-28)23-4-3-11-29-17-23)30-27(35)22-8-6-21(7-9-22)18-34-14-12-33(2)13-15-34/h3-11,16-17,19H,12-15,18H2,1-2H3,(H,30,35)(H,31,32)
|
|||
| InChIKey |
WJEOLQLKVOPQFV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 790299-79-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
15060116, 22490053, 39134542, 75228993, 85246180, 92719832, 99007049, 99436962, 99460877, 109693525, 118844932, 124756972, 124950162, 124950163, 125163777, 125357810, 126620446, 126647233, 126666977, 131326613, 131465129, 134964388, 135261173, 135610416, 135685309, 135685313, 135685317, 135727439, 136922080, 136959519, 137171694, 141745351, 144116186, 152258124, 152344507, 160646963, 162011527, 162037412, 162158733, 162170731, 163123377, 163312260, 163392163, 164826187, 174561053, 176484819, 177748731, 178102284, 180189025, 185990428
|
|||
| ChEBI ID |
CHEBI:63450
|
|||
| SuperDrug ATC ID |
L01XE22
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

