Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M8FD
|
|||
| Former ID |
DCL000994
|
|||
| Drug Name |
SNDX-275
|
|||
| Synonyms |
Entinostat; Histone Deacetylase Inhibitor I; IN1470; MS 275; SNDX 275; MS 27-275; Ms-275; Entinostat (USAN/INN); MS-27-275; Pyridin-3-ylmethyl 4-(2-aminophenylcarbamoyl)benzylcarbamate; Pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate; Pyridin-3-ylmethyl {4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Pyridin-3-ylmethyl{4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Carbamic acid, [[4-[[(2-aminophenyl)amino]carbonyl]phenyl] methyl]-, 3-pyridinylmethyl ester; Carbamic acid, [[4-[[(2-aminophenyl)carbaonyl]phenyl]methyl]-, 3-pyridinylmethyl ester; Entinostat, SNDX-275, MS-27-275, MS-275; N-(2-Aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide; N-(2-aminophenyl)-4-(N-(pyridin-3-ylmethoxycarbonyl)aminomethyl)benzamide; Carbamic acid, ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)-, 3-pyridinylmethyl ester; 3-Pyridinylmethyl ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)carbamate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65; ICD-10: C50, C79.51] | Phase 3 | [1] | |
| Colorectal cancer [ICD-11: 2B91.Z] | Phase 2 | [2] | ||
| Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 2 | [2], [3] | ||
| Non-small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162] | Phase 2 | [2] | ||
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 2 | [2] | ||
| Company |
Bayer Schering
|
|||
| Structure |
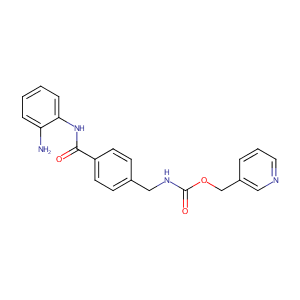 |
Download2D MOL |
||
| Formula |
C21H20N4O3
|
|||
| Canonical SMILES |
C1=CC=C(C(=C1)N)NC(=O)C2=CC=C(C=C2)CNC(=O)OCC3=CN=CC=C3
|
|||
| InChI |
1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26)
|
|||
| InChIKey |
INVTYAOGFAGBOE-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 209783-80-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
529250, 4361630, 8152650, 11442374, 12015232, 14804341, 26758879, 29217590, 29217591, 29223361, 46519433, 50069042, 50875267, 53788084, 53800910, 56312377, 56312987, 57322216, 68529926, 85736423, 87226500, 93309933, 96026018, 99430926, 99436959, 103031574, 103197084, 103846864, 104305887, 117537663, 124756966, 124893190, 125163771, 125333791, 126626762, 126666975, 127325713, 127325714, 127325715, 127325716, 127325717, 128194857, 131305022, 131465123, 134338847, 134339425, 134964213, 135105768, 135314784, 135727398
|
|||
| ChEBI ID |
CHEBI:132082
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03538171) Ph3 Study of Exemestane With or Without Entinostat in Chinese Patients With Hormone Receptor-Positive, Locally Advanced or Metastatic Breast Cancer. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

