Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M6DO
|
|||
| Former ID |
DAP000842
|
|||
| Drug Name |
Aminoglutethimide
|
|||
| Synonyms |
Aminoglutethimidum; Aminoglutetimida; Cytadren; Elipten; Orimeten; Ciba Vision Brand of Aminoglutethimide; Novartis Brand of Aminoglutethimide; A 9657; Ba 16038; AG-1; Aminoglutethimide (AG); Aminoglutethimide [INN:BAN]; Aminoglutethimidum [INN-Latin]; Aminoglutetimida [INN-Spanish]; Ba-16038; C 16038-BA; Cytadren (TN); Dl-Aminoglutethimide; P-Aminoglutethimide; Aminoglutethimide (USP/INN); Glutethimide, para-amino; Alpha-(p-Aminophenyl)-alpha-ethylglutarimide; (+-)-3-(p-Aminophenyl)-3-ethyl-2,6-piperidinedione; (+/-)-p-AMINOGLUTETHIMIDE; (inverted question mark)-p-Aminoglutethimide; 2-(p-Aminophenyl)-2-ethylglutarimide; 3-(4-Aminophenyl)-3-ethyl-2,6-piperidindion; 3-(4-Aminophenyl)-3-ethyl-2,6-piperidinedione; 3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione; 3-(p-Aminophenyl)-3-ethylpiperidine-2,6-dione; 3-Ethyl-3-(p-aminophenyl)-2,6-dioxopiperidine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cushing disease [ICD-11: 5A70] | Approved | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Norvatis Phamaceuticals Corporation
|
|||
| Structure |
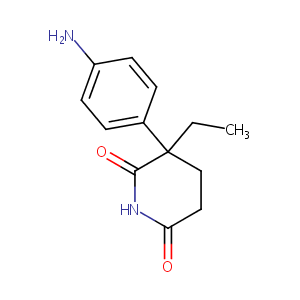 |
Download2D MOL |
||
| Formula |
C13H16N2O2
|
|||
| Canonical SMILES |
CCC1(CCC(=O)NC1=O)C2=CC=C(C=C2)N
|
|||
| InChI |
1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17)
|
|||
| InChIKey |
ROBVIMPUHSLWNV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 125-84-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9819, 459146, 3206291, 4656527, 7847640, 7978687, 8149197, 8151456, 10321343, 10506109, 11336059, 11361298, 11362814, 11365376, 11367938, 11371432, 11373727, 11376100, 11407292, 11462270, 11466272, 11467392, 11483782, 11485983, 11487909, 11490112, 11491959, 11493854, 12012594, 15068844, 17404687, 24278142, 25623233, 26611601, 26679683, 26747446, 26747447, 26747448, 29221324, 46500462, 46506066, 46510177, 47216836, 47291191, 47291192, 47736545, 47810808, 48110505, 48259296, 48334561
|
|||
| ChEBI ID |
CHEBI:2654
|
|||
| ADReCS Drug ID | BADD_D00107 | |||
| SuperDrug ATC ID |
L02BG01
|
|||
| SuperDrug CAS ID |
cas=000125848
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aromatase (CYP19A1) | Target Info | Inhibitor | [3] |
| Cholesterol desmolase (CYP11A1) | Target Info | Inhibitor | [2] | |
| BioCyc | Superpathway of steroid hormone biosynthesis | |||
| Pregnenolone biosynthesis | ||||
| Estradiol biosynthesis II | ||||
| Estradiol biosynthesis I | ||||
| KEGG Pathway | Steroid hormone biosynthesis | |||
| Metabolic pathways | ||||
| Ovarian steroidogenesis | ||||
| NetPath Pathway | FSH Signaling Pathway | |||
| Panther Pathway | Androgen/estrogene/progesterone biosynthesis | |||
| Pathwhiz Pathway | Steroidogenesis | |||
| Androgen and Estrogen Metabolism | ||||
| Reactome | Endogenous sterols | |||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| Metapathway biotransformation | ||||
| Oxidation by Cytochrome P450 | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| Glucocorticoid & Mineralcorticoid Metabolism | ||||
| Corticotropin-releasing hormone | ||||
| Phase 1 - Functionalization of compounds | ||||
| Tryptophan metabolism | ||||
| Ovarian Infertility Genes | ||||
| FSH signaling pathway | ||||
| Integrated Breast Cancer Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7054). | |||
| REF 2 | Breakdown of Th cell immune responses and steroidogenic CYP11A1 expression in CD4+ T cells in a murine model implanted with B16 melanoma. Cell Immunol. 2000 Nov 25;206(1):7-15. | |||
| REF 3 | Aminoglutethimide-induced protein free radical formation on myeloperoxidase: a potential mechanism of agranulocytosis. Chem Res Toxicol. 2007 Jul;20(7):1038-45. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

