Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M5VV
|
|||
| Former ID |
DIB003532
|
|||
| Drug Name |
177Lu-DOTATATE
|
|||
| Indication | Hepatitis C virus infection [ICD-11: 1E51.1; ICD-10: B18.2] | Phase 2 | [1] | |
| Company |
Advanced accelerator applications
|
|||
| Structure |
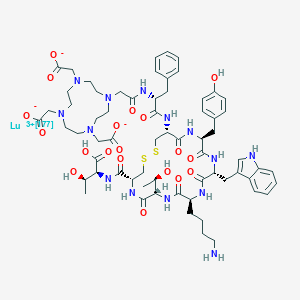 |
Download2D MOL
|
||
| Formula |
C65H87LuN14O19S2
|
|||
| Canonical SMILES |
CC(C1C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)CN6CCN(CCN(CCN(CC6)CC(=O)[O-])CC(=O)[O-])CC(=O)[O-])C(=O)NC(C(C)O)C(=O)O)O.[Lu+3]
|
|||
| InChI |
1S/C65H90N14O19S2.Lu/c1-38(80)56-64(96)73-51(63(95)75-57(39(2)81)65(97)98)37-100-99-36-50(72-59(91)47(28-40-10-4-3-5-11-40)68-52(83)32-76-20-22-77(33-53(84)85)24-26-79(35-55(88)89)27-25-78(23-21-76)34-54(86)87)62(94)70-48(29-41-15-17-43(82)18-16-41)60(92)71-49(30-42-31-67-45-13-7-6-12-44(42)45)61(93)69-46(58(90)74-56)14-8-9-19-66;/h3-7,10-13,15-18,31,38-39,46-51,56-57,67,80-82H,8-9,14,19-30,32-37,66H2,1-2H3,(H,68,83)(H,69,93)(H,70,94)(H,71,92)(H,72,91)(H,73,96)(H,74,90)(H,75,95)(H,84,85)(H,86,87)(H,88,89)(H,97,98);/q;+3/p-3/t38-,39-,46+,47-,48+,49-,50+,51+,56+,57+;/m1./s1/i;1+2
|
|||
| InChIKey |
MXDPZUIOZWKRAA-PRDSJKGBSA-K
|
|||
| CAS Number |
CAS 437608-50-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Somatostatin receptor (SSTR) | Target Info | Modulator | [2] |
| Somatostatin receptor type 4 (SSTR4) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Pathwhiz Pathway | Gastric Acid Production | |||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01860742) Randomized Phase III of PRRT Versus Interferon. U.S. National Institutes of Health. | |||
| REF 2 | Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014 May;43(4):518-25. | |||
| REF 3 | New Drug in Development Shows Improved Progression-Free Survival for Patients with Advanced Metastatic Midgut Neuroendocrine Tumors | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

