Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0LG7X
|
|||
| Drug Name |
RP6530
|
|||
| Synonyms |
tenalisib; HDXDQPRPFRKGKZ-INIZCTEOSA-N; Tenalisib; 1639417-53-0; UNII-2261HH611H; 2261HH611H; Tenalisib [INN]; GTPL9907; SCHEMBL16279460; AKOS027325582; RP-6530; RP 6530; CS-6375; HY-17645; 4H-1-Benzopyran-4-one, 3-(3-fluorophenyl)-2-((1S)-1-(9H-purin-6-ylamino)propyl)-; (S)-2-(1-((7H-purin-6-yl)amino)propyl)-3-(3-fluorophenyl)-4H-chromen-4-one; 3-(3-fluorophenyl)-2-[(1S)-1-[(9H-purin-6-yl)amino]propyl]-4H-chromen-4-one; 3-(3-Fluorophenyl)-2-((1S)-1-((7H-purin-6-yl)amino)propyl)-4H-1-benzopyran-4-one; 1693773-94-2
Click to Show/Hide
|
|||
| Indication | Chronic lymphocytic leukaemia [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 2 | [1] | |
| Cutaneous T-cell lymphoma [ICD-11: 2B01; ICD-10: C84.8; ICD-9: 202.1, 202.2] | Phase 1 | [2], [3] | ||
| Company |
Rhizen PharmaceuticalsLa Chaux-de-Fonds, Switzerland
|
|||
| Structure |
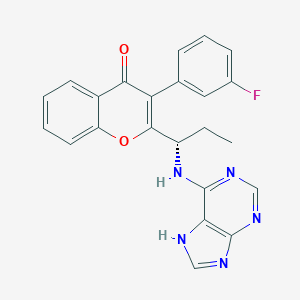 |
Download2D MOL |
||
| Formula |
C23H18FN5O2
|
|||
| Canonical SMILES |
CCC(C1=C(C(=O)C2=CC=CC=C2O1)C3=CC(=CC=C3)F)NC4=NC=NC5=C4NC=N5
|
|||
| InChI |
1S/C23H18FN5O2/c1-2-16(29-23-19-22(26-11-25-19)27-12-28-23)21-18(13-6-5-7-14(24)10-13)20(30)15-8-3-4-9-17(15)31-21/h3-12,16H,2H2,1H3,(H2,25,26,27,28,29)/t16-/m0/s1
|
|||
| InChIKey |
HDXDQPRPFRKGKZ-INIZCTEOSA-N
|
|||
| CAS Number |
CAS 1639417-53-0
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04204057) Efficacy and Safety of Tenalisib (RP6530) in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

