Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0L8ZX
|
|||
| Former ID |
DIB002094
|
|||
| Drug Name |
OX-NLA
|
|||
| Synonyms |
NLA; BLX-NLA; NCX-1510; NO-donating therapeutics, Biolipox; Anti-allergy drug, NicOx/Biolipox; NO-donating allergy therapeutics, Biolipox/Nicox; NO-donating respiratory therapeutics, NicOx/Biolipox; NO-donating therapeutics, Biolipox/Nicox; Liposomal nitric oxide-donating cetirizine derivative (nasal, rhinitis), Biolipox; Nitric oxide-donating cetirizine derivative (liposomal, intranasal, rhinitis), Orexo; OX-NLA (nasal/liposomal formulation, allergic rhinitis/rhinitis); OX-NLA (nasal/liposomal formulation, allergic rhinitis/rhinitis), Meda
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergic rhinitis [ICD-11: CA08.0] | Discontinued in Phase 3 | [1] | |
| Company |
NicOx SA
|
|||
| Structure |
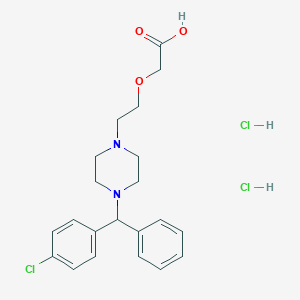 |
Download2D MOL
|
||
| Formula |
C21H27Cl3N2O3
|
|||
| Canonical SMILES |
C1CN(CCN1CCOCC(=O)O)C(C2=CC=CC=C2)C3=CC=C(C=C3)Cl.Cl.Cl
|
|||
| InChI |
1S/C21H25ClN2O3.2ClH/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26;;/h1-9,21H,10-16H2,(H,25,26);2*1H
|
|||
| InChIKey |
PGLIUCLTXOYQMV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 83881-52-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
79563, 583403, 607070, 841137, 3134013, 5432030, 7848621, 7889419, 8145294, 8154475, 10537141, 14843290, 17389810, 24698070, 24859106, 24861915, 24897435, 24897513, 26752862, 29225802, 46392458, 46508218, 48130882, 48414086, 48424424, 49748196, 49825821, 49835454, 49857515, 56310850, 56311443, 56312339, 56312506, 56312529, 56370060, 57288437, 57323764, 57649420, 77411102, 85088003, 87573419, 88575942, 92117615, 92711073, 102851767, 103101651, 103173439, 103932809, 104313279, 104652675
|
|||
| ChEBI ID |
CHEBI:3562
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine receptor (HR) | Target Info | Antagonist | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020184) | |||
| REF 2 | Clinical pipeline report, company report or official report of Orexo. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

