Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0KH8Q
|
|||
| Drug Name |
Ruxolitinib
|
|||
| Synonyms |
Ruxolitinib (JAK inhibitor)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Myelofibrosis [ICD-11: 2A22] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 3 | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Incyte
|
|||
| Structure |
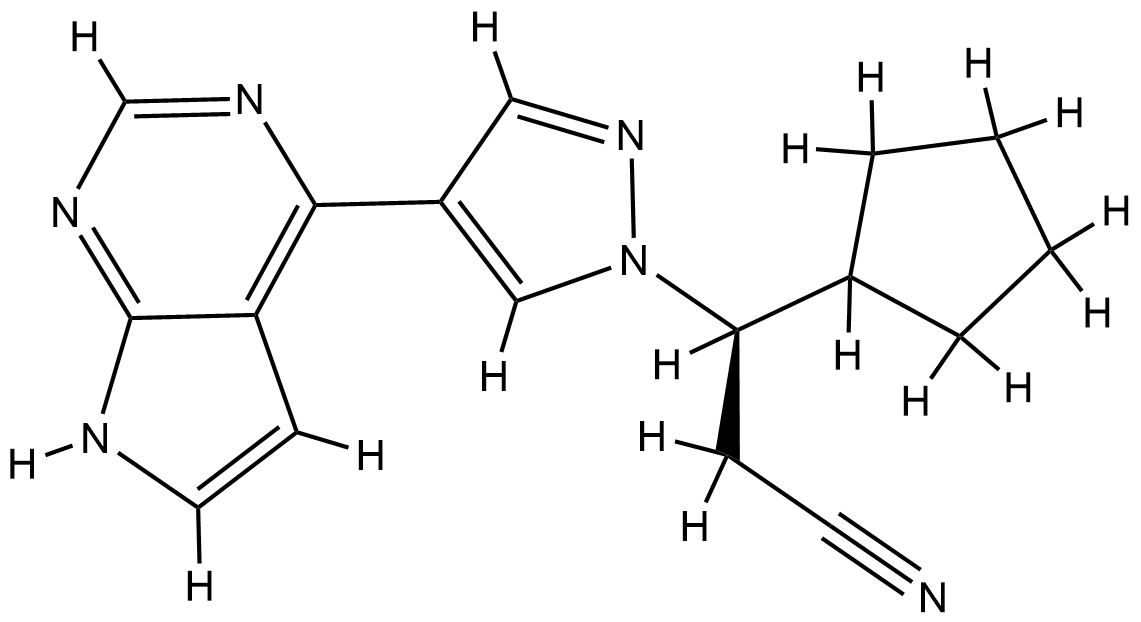 |
Download2D MOL |
||
| Formula |
C17H18N6
|
|||
| Canonical SMILES |
C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3
|
|||
| InChI |
1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1
|
|||
| InChIKey |
HFNKQEVNSGCOJV-OAHLLOKOSA-N
|
|||
| CAS Number |
CAS 941678-49-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
81071277, 85921742, 104253171, 131480859, 134339114, 134449640, 135263142, 135626684, 135693443, 135727401, 136367406, 136378506, 136920365, 136959525, 137168066, 137237509, 137275864, 137875640, 144116074, 152212763, 152258834, 152344048, 152344083, 160645455, 160647685, 162011537, 162038073, 162197164, 163913900, 164193924, 164764917, 165238055, 170502151, 172919539, 174006685, 174474441, 175267965, 175427129, 177748964, 178102315, 186005338, 188376260, 198992749, 208265517, 223617460, 223723945, 224411802, 226532797, 247725985, 249737056
|
|||
| ChEBI ID |
CHEBI:66919
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN janus kinase 1 (JAK-1) | Target Info | Inhibitor | [3], [4] |
| HUMAN janus kinase 2 (JAK-2) | Target Info | Inhibitor | [3], [4] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Incyte begins Phase III trial of ruxolitinib to treat Covid-19. 20.April.2020. | |||
| REF 3 | The Use of Anti-Inflammatory Drugs in the Treatment of People With Severe Coronavirus Disease 2019 (COVID-19): The Perspectives of Clinical Immunologists From China. Clin Immunol. 2020 May;214:108393. | |||
| REF 4 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 202192 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

