Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0KG3Y
|
|||
| Drug Name |
Imatinib
|
|||
| Synonyms |
Cgp 57148; Glamox; Glamox (TN); Gleevec (TN); Glivec (TN); Imatinib (INN); Imatinib (STI571); Imatinib Methansulfonate; Imatinib [INN:BAN]; 112GI019; 152459-95-5; BKJ8M8G5HI; CCRIS 9076; CGP-57148; CHEMBL941; Imatinib free base; STI; UNII-BKJ8M8G5HI
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic myeloid leukaemia [ICD-11: 2A20; ICD-10: C92.7; ICD-9: 205.1] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 3 | [2] | ||
| Middle East Respiratory Syndrome (MERS) [ICD-11: 1D64] | Investigative | [3] | ||
| Severe acute respiratory syndrome (SARS) [ICD-11: 1D65] | Investigative | [3] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Novartis AG
|
|||
| Structure |
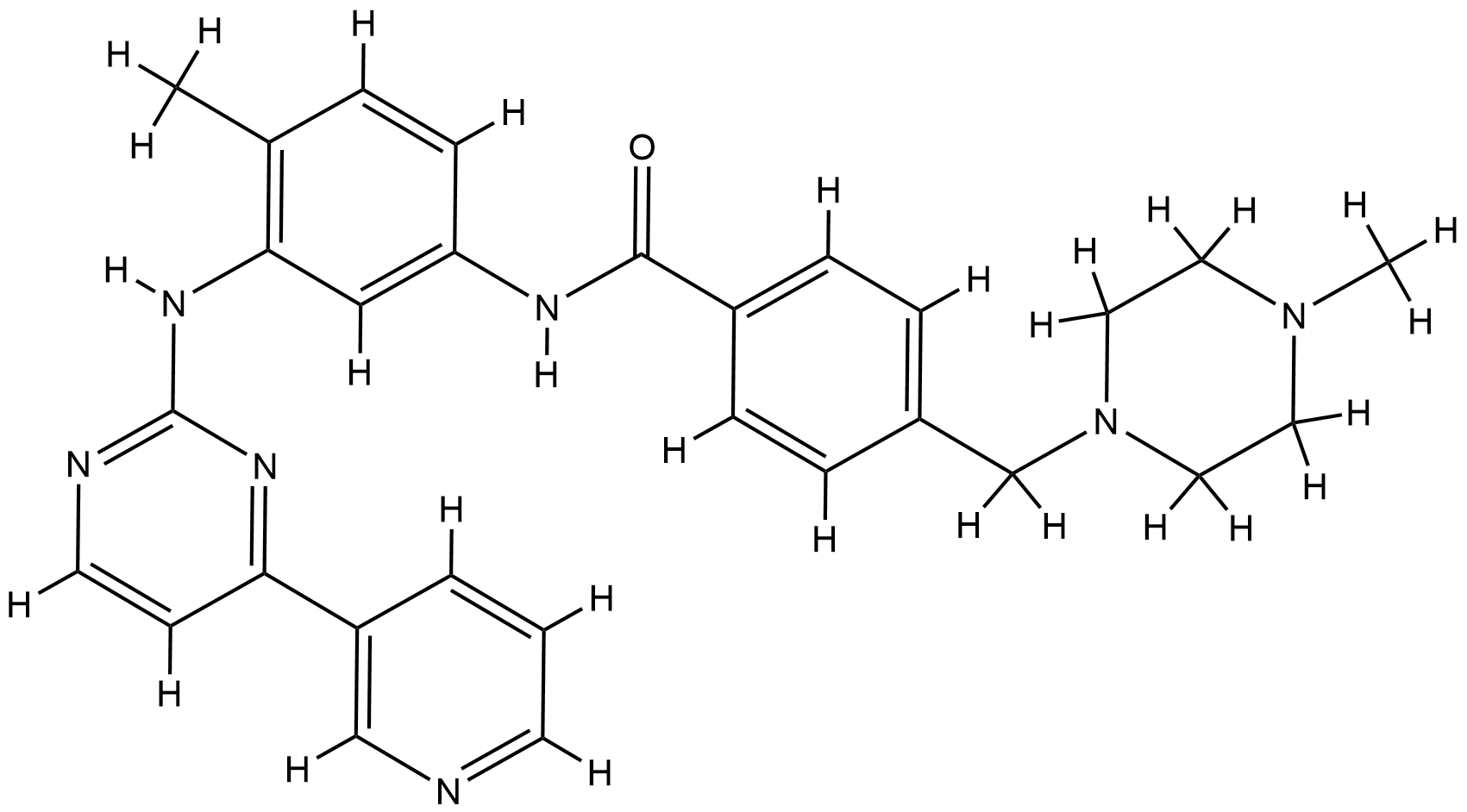 |
Download2D MOL |
||
| Formula |
C29H31N7O
|
|||
| Canonical SMILES |
CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5
|
|||
| InChI |
1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34)
|
|||
| InChIKey |
KTUFNOKKBVMGRW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 152459-95-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
584799, 822644, 828861, 832827, 841977, 5619104, 7890613, 7979593, 8153249, 14859628, 22394533, 24424247, 26697112, 26737110, 29215405, 29215406, 29224346, 46392211, 46393540, 46505055, 46507948, 46513933, 49655235, 50066026, 50070642, 50100104, 50109856, 50353059, 53788935, 53799240, 56311252, 56311284, 56311359, 56311779, 56311988, 56312022, 56312838, 56313109, 56313183, 56313522, 56313562, 56314521, 57288246, 57288452, 57288559, 57288780, 57322698, 57551951, 57578266, 85171056
|
|||
| ChEBI ID |
CHEBI:45783
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN fusion protein Bcr-Abl (Bcr-Abl) | Target Info | Inhibitor | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 078340. | |||
| REF 2 | ClinicalTrials.gov (NCT04356495) Treatments to Decrease the Risk of Hospitalization or Death in Elderly Outpatients With Symptomatic SARS-CoV-2 Infection (COVID-19). U.S. National Institutes of Health. | |||
| REF 3 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

