Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0K8IX
|
|||
| Former ID |
DNC001431
|
|||
| Drug Name |
Thiazolidinedione
|
|||
| Synonyms |
2295-31-0; 1,3-Thiazolidine-2,4-dione; thiazolidine-2,4-dione; 2,4-Dioxothiazolidine; 2,4(3H,5H)-Thiazoledione; USAF EK-5496; Thiazolidindione; UNII-AA68LXK93C; Thiazolidinedione-2,4; NSC 6745; EINECS 218-941-2; BRN 0110700; AA68LXK93C; AI3-61185; CHEBI:50992; NSC6745; ZOBPZXTWZATXDG-UHFFFAOYSA-N; MFCD00005478; 2,4-Thiazolidinedione, 99%; C3H3NO2S; thiazolidine-dione; 2,4-thiazolidindione; 2,5H)-Thiazoledione; PubChem17487
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Approved | [1] | |
| Structure |
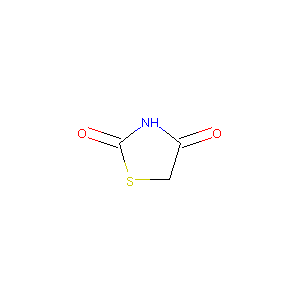 |
Download2D MOL |
||
| Formula |
C3H3NO2S
|
|||
| Canonical SMILES |
C1C(=O)NC(=O)S1
|
|||
| InChI |
1S/C3H3NO2S/c5-2-1-7-3(6)4-2/h1H2,(H,4,5,6)
|
|||
| InChIKey |
ZOBPZXTWZATXDG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 2295-31-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
72705, 609107, 3136030, 5646729, 8153347, 10514447, 14747535, 24863353, 29224487, 36658754, 37339821, 37454049, 50052212, 53790560, 56311219, 56394927, 57322779, 57802988, 72264596, 78732118, 81041520, 87577683, 87917067, 92720016, 93373964, 96107609, 103289393, 104309239, 104668315, 117671506, 117687045, 118053742, 124526817, 125345461, 126576294, 126603281, 126620337, 126658467, 126668350, 126734391, 127114530, 127304974, 127304975, 127304976, 127304977, 127304978, 127304979, 127304980, 127304981, 127304982
|
|||
| ChEBI ID |
CHEBI:50992
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Thiazolidinediones: a comparative review of approved uses. Diabetes Technol Ther. 2000 Autumn;2(3):429-40. | |||
| REF 2 | Functional PPAR-gamma receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med. 2002 Nov;8(11):1281-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

