Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0K0MW
|
|||
| Former ID |
DAP000064
|
|||
| Drug Name |
Lofexidine
|
|||
| Synonyms |
Britlofex; Lofexidina; Lofexidinum; Britlofex (TN); Lofexidina [INN-Spanish]; Lofexidine (INN); Lofexidine [INN:BAN]; Lofexidinum [INN-Latin]; 1H-Imidazole, 2-(1-(2,6-dichlorophenoxy)ethyl)-4,5-dihydro-(9CI); 2-(1-(2,6-Dichlorophenoxy)ethyl)-4,5-dihydro-1H-imidazole; 2-(a-(2,6-dichlorophenoxy)ethyl)2-imidazoline; 2-(alpha-(2,6-Dichlorophenoxy)ethyl)2-imidazoline; 2-(alpha-(2,6-dichlorophenoxy)ethyl) delta-2-imidazoline; 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole; 2-{1-[(2,6-dichlorophenyl)oxy]ethyl}-4,5-dihydro-1H-imidazole
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Heroin and opiate withdrawal [ICD-11: 6C43; ICD-10: F10-F19; ICD-9: 304] | Approved | [1] | |
| Opioid dependence [ICD-11: 6C43.2Z; ICD-10: F11.3] | Approved | [2] | ||
| Therapeutic Class |
Antihypertensive Agents
|
|||
| Company |
US WorldMeds
|
|||
| Structure |
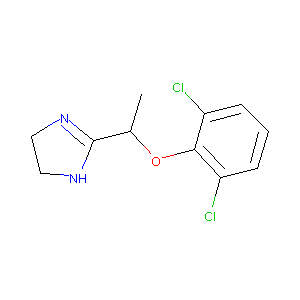 |
Download2D MOL |
||
| Formula |
C11H12Cl2N2O
|
|||
| Canonical SMILES |
CC(C1=NCCN1)OC2=C(C=CC=C2Cl)Cl
|
|||
| InChI |
1S/C11H12Cl2N2O/c1-7(11-14-5-6-15-11)16-10-8(12)3-2-4-9(10)13/h2-4,7H,5-6H2,1H3,(H,14,15)
|
|||
| InChIKey |
KSMAGQUYOIHWFS-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 31036-80-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4955007, 7979787, 8171702, 14774610, 34672885, 46508453, 48416180, 49980645, 56464309, 57310968, 77865548, 91614206, 92309039, 96024831, 103182297, 103930569, 104308830, 117466455, 128428177, 131309952, 134224363, 134337392, 134357768, 135023284, 137069359, 142970890, 152039684, 160967834, 164811810, 170466275, 172876170, 174560267, 175611898, 176484253, 179150300, 179499877, 184643900, 224374099, 226432808, 241060023, 252448593
|
|||
| ChEBI ID |
CHEBI:51368
|
|||
| SuperDrug ATC ID |
N07BC04
|
|||
| SuperDrug CAS ID |
cas=031036803
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides fragilis ATCC43859
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Lofexidine can be metabolized by Bacteroides fragilis ATCC43859 (log2FC = -0.341; p = 0.041). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor Alpha-2 (ADRA2) | Target Info | Agonist | [1], [4] |
| Adrenergic receptor alpha-2A (ADRA2A) | Target Info | Agonist | [2] | |
| KEGG Pathway | cGMP-PKG signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Panther Pathway | Alpha adrenergic receptor signaling pathway | |||
| Reactome | Adrenoceptors | |||
| Adrenaline signalling through Alpha-2 adrenergic receptor | ||||
| Adrenaline,noradrenaline inhibits insulin secretion | ||||
| G alpha (i) signalling events | ||||
| G alpha (z) signalling events | ||||
| Surfactant metabolism | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Platelet Aggregation (Plug Formation) | ||||
| Integration of energy metabolism | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | A Phase 3 placebo-controlled, double-blind, multi-site trial of the alpha-2-adrenergic agonist, lofexidine, for opioid withdrawal. Drug Alcohol Depend. 2008 Sep 1;97(1-2):158-68. | |||
| REF 2 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||
| REF 3 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||
| REF 4 | Clinical pharmacokinetics of lofexidine, the alpha 2-adrenergic receptor agonist, in opiate addicts plasma using a highly sensitive liquid chromatography tandem mass spectrometric analysis. Am J DrugAlcohol Abuse. 2008;34(5):611-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

