Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J6RQ
|
|||
| Former ID |
DNC008724
|
|||
| Drug Name |
1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one
|
|||
| Synonyms |
3654-49-7; (1E,4E)-1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one; 1,5-Bis(4-hydroxyphenyl)penta-1,4-dien-3-one; Bis-1,5-(4-Hydroxyphenyl)-1,4-Pentadien-3-one; 1,5-Bis-(4-hydroxyphenyl)-1,4-pentadien-3-one; CHEMBL129134; EINECS 222-896-4; AC1O5NFJ; 1,5-Di(p-hydroxyphenyl)-1,4-pentadien-3-one; 1,5-BIS(4-HYDROXYPHENYL)-1,4-PENTADIEN-3-ONE; 4,4'-Dihydroxydistyrylketon; ZINC6092599; BDBM50067044; AKOS015962226; CD-1056; M064; CC-03405; AC-16155; A823289; C-35230; 654B497; 1,5-Bis-(4-hydroxy-phenyl)-penta-1,4-dien-3-one; W-106603
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
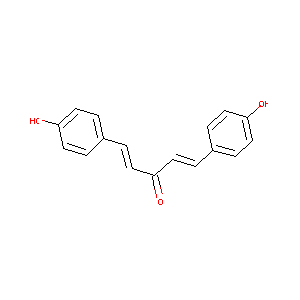 |
Download2D MOL |
||
| Formula |
C17H14O3
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C=CC(=O)C=CC2=CC=C(C=C2)O)O
|
|||
| InChI |
1S/C17H14O3/c18-15-7-1-13(2-8-15)5-11-17(20)12-6-14-3-9-16(19)10-4-14/h1-12,18-19H/b11-5+,12-6+
|
|||
| InChIKey |
FTEGUKWEUQPKIS-YDWXAUTNSA-N
|
|||
| CAS Number |
CAS 3654-49-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Debrisoquine 4-hydroxylase (CYP2D6) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Metabolism of xenobiotics by cytochrome P450 | |||
| Drug metabolism - cytochrome P450 | ||||
| Serotonergic synapse | ||||
| Reactome | Xenobiotics | |||
| WikiPathways | Metapathway biotransformation | |||
| Tamoxifen metabolism | ||||
| Oxidation by Cytochrome P450 | ||||
| Vitamin D Receptor Pathway | ||||
| Aripiprazole Metabolic Pathway | ||||
| Fatty Acid Omega Oxidation | ||||
| Codeine and Morphine Metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Structure-activity relationships for the inhibition of recombinant human cytochromes P450 by curcumin analogues. Eur J Med Chem. 2008 Aug;43(8):1621-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

