Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J5YC
|
|||
| Former ID |
DAP000520
|
|||
| Drug Name |
Fosphenytoin
|
|||
| Synonyms |
Fosfenitoina; Fosphenytoine; Fosphenytoinum; HMPDP; Prodilantin; ACC-9653; Cerebyx (TN); Fosfenitoina [INN-Spanish]; Fosphenytoin (INN); Fosphenytoin [INN:BAN]; Fosphenytoine [INN-French]; Fosphenytoinum [INN-Latin]; Prodilantin (TN); (2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl dihydrogen phosphate; (3-Phosphoryloxymethyl)phenytoin; 3-(hydroxymethyl)phenytoin disodium phosphate; 3-(hydroxymethyl)phenytoin phosphate ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68; ICD-10: G40] | Approved | [1] | |
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
Pfizer Pharmaceuticals
|
|||
| Structure |
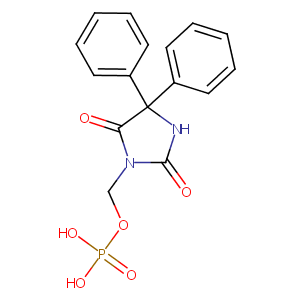 |
Download2D MOL |
||
| Formula |
C16H15N2O6P
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C2(C(=O)N(C(=O)N2)COP(=O)(O)O)C3=CC=CC=C3
|
|||
| InChI |
1S/C16H15N2O6P/c19-14-16(12-7-3-1-4-8-12,13-9-5-2-6-10-13)17-15(20)18(14)11-24-25(21,22)23/h1-10H,11H2,(H,17,20)(H2,21,22,23)
|
|||
| InChIKey |
XWLUWCNOOVRFPX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 93390-81-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10042, 6866976, 7979320, 8184645, 14779124, 43114191, 46505168, 50065395, 50070875, 57313837, 79614497, 93166378, 96024687, 104309885, 117621485, 124955679, 126420857, 129751033, 134222960, 134337812, 135079341, 137004943, 142774087, 160964618, 162179214, 170465016, 175267433, 178103765, 179117051, 223439760, 226471877, 252350071
|
|||
| ChEBI ID |
CHEBI:5165
|
|||
| ADReCS Drug ID | BADD_D00970 ; BADD_D00971 | |||
| SuperDrug ATC ID |
N03AB05
|
|||
| SuperDrug CAS ID |
cas=093390819
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated sodium channel alpha Nav1.5 (SCN5A) | Target Info | Blocker | [2] |
| KEGG Pathway | Adrenergic signaling in cardiomyocytes | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Cardiac Progenitor Differentiation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Lacosamide: a new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs. 2009;23(7):555-68. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

