Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0IV4C
|
|||
| Former ID |
DIB014357
|
|||
| Drug Name |
WP-1066
|
|||
| Synonyms |
WP1066; 857064-38-1; (S,E)-3-(6-Bromopyridin-2-yl)-2-cyano-N-(1-phenylethyl)acrylamide; WP 1066; UNII-63V8AIE65T; 63V8AIE65T; WP-1066; AK-99218; C17H14BrN3O; (E)-3-(6-bromopyridin-2-yl)-2-cyano-N-[(1S)-1-phenylethyl]prop-2-enamide; MLS006010178; SCHEMBL1315826; QCR-16; SCHEMBL1315831; GTPL7972; CHEMBL1923234; EX-A760; AOB1497; DTXSID50235007; MolPort-044-723-708; MolPort-023-219-149; ZINC13983221; AKOS016007983; WP1066/WP-1066; CS-2736; DB12679; 2-Propenamide, 3-(6-bromo-2-pyridinyl)-2-cyano-N-((1S)-1-phenylethyl)-, (2E)-; HY-15312
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Recurrent glioblastoma [ICD-11: 2A00.00; ICD-10: C71] | Phase 1/2 | [1] | |
| Brain cancer [ICD-11: 2A00] | Phase 1 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [3], [4] | ||
| Company |
MD Anderson Cancer Center
|
|||
| Structure |
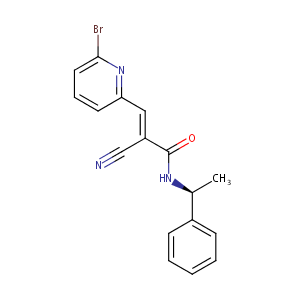 |
Download2D MOL |
||
| Formula |
C17H14BrN3O
|
|||
| Canonical SMILES |
CC(C1=CC=CC=C1)NC(=O)C(=CC2=NC(=CC=C2)Br)C#N
|
|||
| InChI |
1S/C17H14BrN3O/c1-12(13-6-3-2-4-7-13)20-17(22)14(11-19)10-15-8-5-9-16(18)21-15/h2-10,12H,1H3,(H,20,22)/b14-10+/t12-/m0/s1
|
|||
| InChIKey |
VFUAJMPDXIRPKO-LQELWAHVSA-N
|
|||
| CAS Number |
CAS 857064-38-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
16292933, 23395262, 42290123, 53789784, 80274042, 135351928, 136349981, 136925154, 136946471, 140830160, 152159603, 162012023, 162201843, 163403336, 163642736, 163846895, 164043522, 164194013, 172919879, 184816677, 189561502, 198969184, 210275216, 210280855, 223381468, 223573724, 223704784, 227538911, 227538916, 249565655, 249823448, 250182047, 250211322, 252056542, 252160914, 252439924, 252450208
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7972). | |||
| REF 4 | ClinicalTrials.gov (NCT01904123) A Phase I Trial of WP1066. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

