Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0IT6Q
|
|||
| Former ID |
DCL000367
|
|||
| Drug Name |
Tandutinib
|
|||
| Synonyms |
CT 53518; CT53518; MLN 518; CT-53518; MLN-0518; MLN-518; MLN518, CT53518; Tandutinib (USAN/INN); (4-(6-Methoxy-7-(3-piperidylpropoxy)quinazolin-4-yl)piperazinyl)-N-(4-(methylethoxy)phenyl)carboxamide; 4-(6-Methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl)piperazine-1-carboxylic acid (4-isopropoxyphenyl)amide; 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-(4-propan-2-yloxyphenyl)piperazine-1-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anaplastic mixed oligoastrocytoma [ICD-11: 2A00.0Y; ICD-10: C71; ICD-9: 191] | Discontinued in Phase 2 | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Millenium; Takeda
|
|||
| Structure |
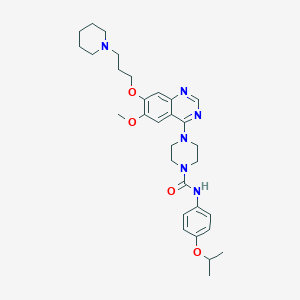 |
Download2D MOL |
||
| Formula |
C31H42N6O4
|
|||
| Canonical SMILES |
CC(C)OC1=CC=C(C=C1)NC(=O)N2CCN(CC2)C3=NC=NC4=CC(=C(C=C43)OC)OCCCN5CCCCC5
|
|||
| InChI |
1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38)
|
|||
| InChIKey |
UXXQOJXBIDBUAC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 387867-13-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:90237
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fms-like tyrosine kinase 3 (FLT-3) | Target Info | Inhibitor | [3], [4] |
| KEGG Pathway | Cytokine-cytokine receptor interaction | |||
| Hematopoietic cell lineage | ||||
| Pathways in cancer | ||||
| Transcriptional misregulation in cancer | ||||
| Acute myeloid leukemia | ||||
| Central carbon metabolism in cancer | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5695). | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017148) | |||
| REF 3 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
| REF 4 | Clinical pipeline report, company report or official report of Takeda (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

