Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I8DD
|
|||
| Former ID |
DAP000499
|
|||
| Drug Name |
Dantrolene
|
|||
| Synonyms |
Dantrium; Dantroleno; Dantrolenum; DANTROLENE SODIUM; Dantrium Intravenous; Dantrium (TN); Dantrolen (TN); Dantroleno [INN-Spanish]; Dantrolenum [INN-Latin]; F-368; Dantrolene (USAN/INN); Dantrolene [USAN:BAN:INN]; 1-(((5-(4-Nitrophenyl)-2-furanyl)methylene)amino)-2,4-imidazolidinedione; 1-((5-(p-Nitrophenyl)furfurylidene)amino)hydantoin; 1-({(1E)-[5-(4-nitrophenyl)furan-2-yl]methylidene}amino)imidazolidine-2,4-dione; 1-({[5-(4-nitrophenyl)furan-2-yl]methylidene}amino)imidazolidine-2,4-dione; 1-[(E)-[5-(4-nitrophenyl)furan-2-yl]methylideneamino]imidazolidine-2,4-dione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hyperthermia [ICD-11: MG26; ICD-9: 995.86] | Approved | [1], [2] | |
| Therapeutic Class |
Muscle Relaxants
|
|||
| Company |
Procter & Gamble
|
|||
| Structure |
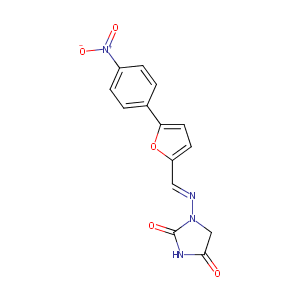 |
Download2D MOL |
||
| Formula |
C14H10N4O5
|
|||
| Canonical SMILES |
C1C(=O)NC(=O)N1N=CC2=CC=C(O2)C3=CC=C(C=C3)[N+](=O)[O-]
|
|||
| InChI |
1S/C14H10N4O5/c19-13-8-17(14(20)16-13)15-7-11-5-6-12(23-11)9-1-3-10(4-2-9)18(21)22/h1-7H,8H2,(H,16,19,20)/b15-7+
|
|||
| InChIKey |
OZOMQRBLCMDCEG-VIZOYTHASA-N
|
|||
| CAS Number |
CAS 7261-97-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9154, 7849406, 7979024, 14899016, 23950769, 26756482, 26756773, 43526625, 46504976, 47573046, 47573478, 47646247, 47646248, 47869294, 47869295, 48018557, 48018558, 48019033, 48019034, 48243503, 48415841, 49977300, 50111415, 50824672, 53789979, 66409319, 85787712, 85788469, 92309721, 92310311, 93166345, 96079574, 99001539, 99300635, 99302153, 104234349, 114780474, 121403951, 124892433, 124899355, 134337792, 134987695, 137005597, 137142472, 160964552, 164788314, 175268146, 179038874, 179113862, 226426040
|
|||
| ADReCS Drug ID | BADD_D00577 | |||
| SuperDrug ATC ID |
M03CA01
|
|||
| SuperDrug CAS ID |
cas=007261974
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [3] | |||
| Experimental Method | High-throughput screening | |||
| Description | Dantrolene can be metabolized by gut microbiota. | |||
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Odoribacter splanchnicus
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Odoribacter splanchnicus was decreased by Dantrolene sodium salt (adjusted p-values: 2.63E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Ryanodine receptor (RYR) | Target Info | Modulator | [5] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4172). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076856. | |||
| REF 3 | Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell. 2020 Jun 25;181(7):1661-1679.e22. | |||
| REF 4 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

