Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I2AG
|
|||
| Former ID |
DNC004389
|
|||
| Drug Name |
S-(N-4bromophenyl-N-hydroxycarbamoyl)glutathione
|
|||
| Synonyms |
CHEMBL128872; AC1L9LM2; S-(N-4bromophenyl-N-hydroxycarbamoyl)glutathione; SCHEMBL3281589; BDBM50092826; L-gammaGlu-S-[Hydroxy(4-bromophenyl)carbamoyl]-L-Cys-Gly-OH; (2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydroxy)amino]carbonyl}thio)methyl]-2-(carboxyamino)-2-oxoethyl]amino}-5-oxopentanoic acid; (S)-2-amino-5-((R)-3-((4-bromophenyl)hydroxycarbamoylthio)-1-(carboxymethylamino)-1-oxopropan-2-ylamino)-5-oxopentanoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
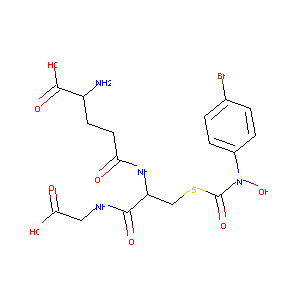 |
Download2D MOL |
||
| Formula |
C17H21BrN4O8S
|
|||
| Canonical SMILES |
C1=CC(=CC=C1N(C(=O)SCC(C(=O)NCC(=O)O)NC(=O)CCC(C(=O)O)N)O)Br
|
|||
| InChI |
1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1
|
|||
| InChIKey |
IQPLOQWSCZRUCA-RYUDHWBXSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Lactoylglutathione lyase (GLO1) | Target Info | Inhibitor | [1] |
| BioCyc | Methylglyoxal degradation I | |||
| KEGG Pathway | Pyruvate metabolism | |||
| NetPath Pathway | TCR Signaling Pathway | |||
| Pathwhiz Pathway | Pyruvaldehyde Degradation | |||
| Pyruvate Metabolism | ||||
| Reactome | Pyruvate metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Role of hydrophobic interactions in binding S-(N-aryl/alkyl-N-hydroxycarbamoyl)glutathiones to the active site of the antitumor target enzyme glyoxalase I. J Med Chem. 2000 Oct 19;43(21):3981-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

