Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0HJ5H
|
|||
| Former ID |
DIB007684
|
|||
| Drug Name |
BGC-20-1531
|
|||
| Synonyms |
AH-22921; EP4 antagonist, BTG; EP4 antagonist, Pharmagene; Migraine therapy, BTG; Migraine therapy, Pharmagene; PGN-1531; EP4 receptor antagonists (migraine), Asterand; R-4 (migraine), Pharmagene
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Phase 2 | [1], [2] | |
| Company |
Pharmagene plc
|
|||
| Structure |
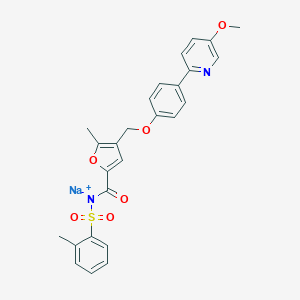 |
Download2D MOL
|
||
| Formula |
C26H23N2NaO6S
|
|||
| Canonical SMILES |
CC1=CC=CC=C1S(=O)(=O)[N-]C(=O)C2=CC(=C(O2)C)COC3=CC=C(C=C3)C4=NC=C(C=C4)OC.[Na+]
|
|||
| InChI |
1S/C26H24N2O6S.Na/c1-17-6-4-5-7-25(17)35(30,31)28-26(29)24-14-20(18(2)34-24)16-33-21-10-8-19(9-11-21)23-13-12-22(32-3)15-27-23;/h4-15H,16H2,1-3H3,(H,28,29);/q;+1/p-1
|
|||
| InChIKey |
TVDHMDXPKGMXRT-UHFFFAOYSA-M
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostaglandin E2 receptor EP4 (PTGER4) | Target Info | Antagonist | [3] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Inflammatory mediator regulation of TRP channels | ||||
| Renin secretion | ||||
| Pathways in cancer | ||||
| NetPath Pathway | FSH Signaling Pathway | |||
| IL2 Signaling Pathway | ||||
| IL4 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | ||||
| Reactome | Prostanoid ligand receptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Vitamin D Receptor Pathway | ||||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3380). | |||
| REF 2 | ClinicalTrials.gov (NCT00888680) Double-blind, Placebo-controlled Study of BGC20-1531 in Migraine. U.S. National Institutes of Health. | |||
| REF 3 | BGC20-1531, a novel, potent and selective prostanoid EP4 receptor antagonist: a putative new treatment for migraine headache. Br J Pharmacol. 2009 January; 156(2): 316-327. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

