Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0H0ND
|
|||
| Former ID |
DAP000552
|
|||
| Drug Name |
Simvastatin
|
|||
| Synonyms |
Cholestat; Coledis; Colemin; Corolin; Denan; Labistatin; Lipex; Lipovas; Lodales; Medipo; Nivelipol; Pantok; Rendapid; Simovil; Simvastatina; Simvastatine; Simvastatinum; Sinvacor; Sivastin; Synvinolin; Vasotenal; Zocor; Zocord; Simvast CR; Simvastatina [Spanish]; Simvastatine [French]; Simvastatinum [Latin]; MK 0733; MK 733; MK733; TNP00259; DRG-0320; KS-1113; L 644128-000U; MK-0733; MK-733; Simcard (TN); Simlup (TN); Simvacor (TN); Simvastatin & Primycin; Simvastatin, Compactin; Zocor (TN); Simvastatin [USAN:INN:BAN]; Simvastatin (JAN/USP/INN); Zocor, Simlup, Simcard, Simvacor, Simvoget, Zorced, Simvastatin; [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,*aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate; Butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1 alpha,3 alpha,7 beta,8 beta(2S*,4S*),-8a beta; 2,2-Dimethylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; 2,2-Dimethylbutyric acid, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8aR)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypercholesterolaemia [ICD-11: 5C80.0] | Approved | [1], [2] | |
| Therapeutic Class |
Anticholesteremic Agents
|
|||
| Company |
Merck & Co
|
|||
| Structure |
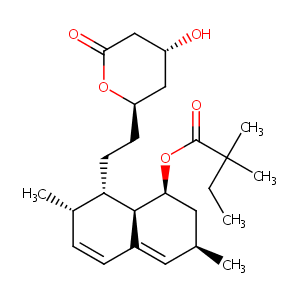 |
Download2D MOL |
||
| Formula |
C25H38O5
|
|||
| Canonical SMILES |
CCC(C)(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O)C
|
|||
| InChI |
1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1
|
|||
| InChIKey |
RYMZZMVNJRMUDD-HGQWONQESA-N
|
|||
| CAS Number |
CAS 79902-63-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
496592, 648581, 7847500, 7980599, 8183649, 10321271, 10852028, 11113242, 11342166, 11362349, 11364611, 11367173, 11369735, 11371657, 11374383, 11377897, 11466893, 11468013, 11485617, 11486566, 11487751, 11489487, 11490485, 11492447, 11495531, 11528633, 11533326, 12013879, 12146013, 14831549, 14929313, 24724617, 25819951, 26612685, 26680673, 26759532, 34718442, 46508654, 47365442, 47440515, 47736737, 47885633, 47885634, 48110715, 48334759, 48416540, 49698671, 50086525, 50100555, 50100556
|
|||
| ChEBI ID |
CHEBI:9150
|
|||
| ADReCS Drug ID | BADD_D02026 | |||
| SuperDrug ATC ID |
C10AA01
|
|||
| SuperDrug CAS ID |
cas=079902639
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Lactobacillus
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Metabolic Effect | Increase activity; Increase side effect (myopathy) | |||
| Description | Simvastatin can be metabolized by Lactobacillus, which results in the increase of drug's activity and side effect (myopathy). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [4], [5], [6] | |||
| Metabolic Reaction | Hydrolysis, demethylation and beta-oxidation | |||
| Resulting Metabolite | Lactic acid, 3-hydroxybutanoic acid, cyclohexanecarboxylic acid; 2-hydroxyvaleric acid | |||
| Metabolic Effect | Decrease activity | |||
| Description | Simvastatin can be metabolized to Lactic acid, 3-hydroxybutanoic acid, cyclohexanecarboxylic acid and 2-hydroxyvaleric acid by gut microbiota through hydrolysis, demethylation and beta-oxidation, which results in the decrease of the drug's activity. | |||
| Studied Microbe: Gut microbiota unspecific | [7], [8], [9], [10] | |||
| Metabolic Reaction | Hydroxylation and ring fission | |||
| Resulting Metabolite | 2-hydroxy simvastatin acid | |||
| Metabolic Effect | Increase activity | |||
| Description | Simvastatin can be metabolized to 2-hydroxy simvastatin acid by gut microbiota through hydroxylation and ring fission, which results in the increase of the drug's activity. | |||
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Parabacteroides distasonis
Show/Hide Hierarchy
|
[11] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Parabacteroides distasonis was decreased by Simvastatin (adjusted p-values: 7.62E-04). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Roseburia intestinalis
Show/Hide Hierarchy
|
[11] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Roseburia intestinalis was decreased by Simvastatin (adjusted p-values: 2.62E-03). | |||
|
Studied Microbe: Ruminococcus torques
Show/Hide Hierarchy
|
[11] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Ruminococcus torques was decreased by Simvastatin (adjusted p-values: 4.02E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HMG-CoA reductase (HMGCR) | Target Info | Inhibitor | [12] |
| BioCyc | Superpathway of geranylgeranyldiphosphate biosynthesis I (via mevalonate) | |||
| Superpathway of cholesterol biosynthesis | ||||
| Mevalonate pathway | ||||
| KEGG Pathway | Terpenoid backbone biosynthesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| AMPK signaling pathway | ||||
| Bile secretion | ||||
| NetPath Pathway | IL5 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| TSH Signaling Pathway | ||||
| Panther Pathway | Cholesterol biosynthesis | |||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| WikiPathways | Statin Pathway | |||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ||||
| Activation of Gene Expression by SREBP (SREBF) | ||||
| SREBF and miR33 in cholesterol and lipid homeostasis | ||||
| Integrated Breast Cancer Pathway | ||||
| SREBP signalling | ||||
| Cholesterol Biosynthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2955). | |||
| REF 2 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||
| REF 3 | Gut microbiota modulates drug pharmacokinetics. Drug Metab Rev. 2018 Aug;50(3):357-368. | |||
| REF 4 | The influence of gut microbiota on drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2016;12(1):31-40. | |||
| REF 5 | Acute liver necrosis following overdose of paracetamol. Br Med J. 1966 Aug 27;2(5512):497-9. | |||
| REF 6 | Gut Microbiome and Response to Cardiovascular Drugs. Circ Genom Precis Med. 2019 Sep;12(9):421-429. | |||
| REF 7 | Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998 Aug;64(2):177-82. | |||
| REF 8 | Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014 Sep;42(9):1508-13. | |||
| REF 9 | Gut Microbiota-Mediated Drug-Antibiotic Interactions. Drug Metab Dispos. 2015 Oct;43(10):1581-9. | |||
| REF 10 | A pharmacokinetic drug-drug interaction model of simvastatin and clarithromycin in humans. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:5703-6. | |||
| REF 11 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 12 | Equally potent inhibitors of cholesterol synthesis in human hepatocytes have distinguishable effects on different cytochrome P450 enzymes. Biopharm Drug Dispos. 2000 Dec;21(9):353-64. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

