Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0F3CO
|
|||
| Former ID |
DNC006043
|
|||
| Drug Name |
KOJIC ACID
|
|||
| Synonyms |
kojic acid; 501-30-4; 5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one; 5-Hydroxy-2-(hydroxymethyl)-4-pyrone; 5-Hydroxy-2-hydroxymethyl-4-pyrone; 4H-PYRAN-4-ONE, 5-HYDROXY-2-(HYDROXYMETHYL)-; 5-hydroxy-2-(hydroxymethyl)pyran-4-one; acido kojico; 2-Hydroxymethyl-5-hydroxy-gamma-pyrone; 2-(Hydroxymethyl)-5-hydroxy-4H-pyran-4-one; UNII-6K23F1TT52; CCRIS 4131; 5-Hydroxy-2-hydroxymethyl-4H-4-pyranone; NSC 1942; EINECS 207-922-4; BRN 0120895; Pyran-4-one, 5-hydroxy-2-(hydroxymethyl); 5-Hydroxy-2-hydroxymethyl-4H-pyran-4-one; AI3-02549
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
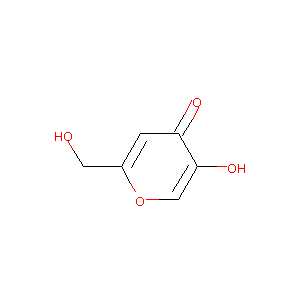 |
Download2D MOL |
||
| Formula |
C6H6O4
|
|||
| Canonical SMILES |
C1=C(OC=C(C1=O)O)CO
|
|||
| InChI |
1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2
|
|||
| InChIKey |
BEJNERDRQOWKJM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 501-30-4
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:43572
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Tyrosinase (TYR) | Target Info | Inhibitor | [1] |
| BioCyc | (S)-reticuline biosynthesis | |||
| Eumelanin biosynthesis | ||||
| L-dopachrome biosynthesis | ||||
| KEGG Pathway | Tyrosine metabolism | |||
| Riboflavin metabolism | ||||
| Metabolic pathways | ||||
| Melanogenesis | ||||
| Pathwhiz Pathway | Riboflavin Metabolism | |||
| Tyrosine Metabolism | ||||
| WikiPathways | Dopamine metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | A novel ring-expanded product with enhanced tyrosinase inhibitory activity from classical Fe-catalyzed oxidation of rosmarinic acid, a potent antio... Bioorg Med Chem Lett. 2010 Dec 15;20(24):7393-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

