Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0F0ZE
|
|||
| Former ID |
DAP001451
|
|||
| Drug Name |
Merimepodib
|
|||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65] | Approved | [1] | |
| Gastric adenocarcinoma [ICD-11: 2B72; ICD-9: 151] | Phase 3 | [1] | ||
| Head and neck cancer [ICD-11: 2D42; ICD-9: 199] | Phase 2 | [1] | ||
| Hepatitis C virus infection [ICD-11: 1E51.1; ICD-10: B18.2] | Patented | [2] | ||
| Hepatitis virus infection [ICD-11: 1E50-1E51; ICD-9: 573.3] | Discontinued in Phase 2 | [3] | ||
| Company |
GSK
|
|||
| Structure |
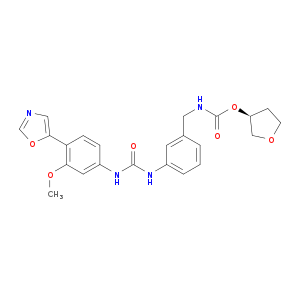 |
Download2D MOL |
||
| Formula |
C23H24N4O6
|
|||
| Canonical SMILES |
COC1=C(C=CC(=C1)NC(=O)NC2=CC=CC(=C2)CNC(=O)OC3CCOC3)C4=CN=CO4
|
|||
| InChI |
1S/C23H24N4O6/c1-30-20-10-17(5-6-19(20)21-12-24-14-32-21)27-22(28)26-16-4-2-3-15(9-16)11-25-23(29)33-18-7-8-31-13-18/h2-6,9-10,12,14,18H,7-8,11,13H2,1H3,(H,25,29)(H2,26,27,28)/t18-/m0/s1
|
|||
| InChIKey |
JBPUGFODGPKTDW-SFHVURJKSA-N
|
|||
| CAS Number |
CAS 198821-22-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
640687, 10251736, 12015083, 14784233, 14857722, 46229554, 47206686, 52470262, 57347884, 103261008, 103998789, 104435748, 128251684, 134223085, 134338650, 134340678, 135110565, 137237685, 137267687, 141966212, 163306660, 175426334, 175426871, 179150134, 184528635, 198954413, 210275043, 210280681, 224157104, 226666702, 242060097, 252479539
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 022059. | |||
| REF 2 | Inosine-5'-monophosphate dehydrogenase (IMPDH) inhibitors: a patent and scientific literature review (2002-2016).Expert Opin Ther Pat. 2017 Jun;27(6):677-690. | |||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008158) | |||
| REF 4 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||
| REF 5 | VX-497: a novel, selective IMPDH inhibitor and immunosuppressive agent. J Pharm Sci. 2001 May;90(5):625-37. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

