Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E6YQ
|
|||
| Former ID |
DAP000558
|
|||
| Drug Name |
Tacrine
|
|||
| Synonyms |
Cognex; Romotal; Tacrina; Tacrinal; Tacrinum; Tenakrin; Tetrahydroaminacrine; Tetrahydroaminoacridine; Tetrahydroaminocrin; Tetrahydroaminocrine; Tha; Tacrine hydrochloride; BBL001044; CS 12602; Cognex (TN); Tacrina [INN-Spanish]; Tacrinal (TN); Tacrine (INN); Tacrine [INN:BAN]; Tacrinum [INN-Latin]; 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE; 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE (SEE ALSO 1684-40-8); 1,2,3,4-Tetrahydro-9-acridineamine; 1,2,3,4-Tetrahydro-acridin-9-ylamine; 1,2,3,4-Tetrahydroaminoacridine; 1,2,3,4-tetrahydroacridin-9-amine; 5-Amino-6,7,8,9-tetrahydroacridine (European); 9-AMINOTETRAHYDROACRIDINE; 9-Acridinamine, 1,2,3,4-tetrahydro-(9CI); 9-Amino-1,2,3,4-Tetrahydroacridine Hydrate Hydrochloride Hydrate; 9-amino-1,2,3,4-tetrahydroacridine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Approved | [1], [2] | |
| Therapeutic Class |
Parasympathomimetics
|
|||
| Structure |
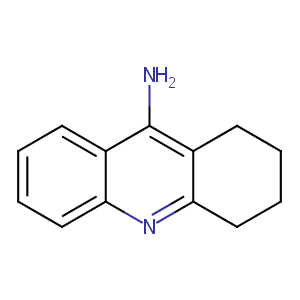 |
Download2D MOL |
||
| Formula |
C13H14N2
|
|||
| Canonical SMILES |
C1CCC2=NC3=CC=CC=C3C(=C2C1)N
|
|||
| InChI |
1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15)
|
|||
| InChIKey |
YLJREFDVOIBQDA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 321-64-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4630, 597209, 3249865, 7423820, 7890757, 7980766, 8151345, 10518487, 10589113, 11110734, 11113824, 11336111, 11361350, 11363310, 11365872, 11368434, 11372617, 11374476, 11376596, 11451154, 11462322, 11466357, 11467477, 11485559, 11485994, 11489628, 11491524, 11492634, 11494230, 15120928, 24339135, 26697094, 26746578, 26751997, 26751998, 29221124, 46392558, 46393827, 46393828, 46505487, 46509736, 47291206, 47365271, 47440346, 47440347, 47515380, 47515381, 47589065, 47736566, 47736567
|
|||
| ChEBI ID |
CHEBI:45980
|
|||
| ADReCS Drug ID | BADD_D02104 | |||
| SuperDrug ATC ID |
N06DA01
|
|||
| SuperDrug CAS ID |
cas=000321642
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Acetylcholinesterase (AChE) | Target Info | Inhibitor | [3] |
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Cholinergic synapse | ||||
| Panther Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | ||||
| Pathwhiz Pathway | Phospholipid Biosynthesis | |||
| Pathway Interaction Database | ATF-2 transcription factor network | |||
| WikiPathways | Monoamine Transport | |||
| Biogenic Amine Synthesis | ||||
| Acetylcholine Synthesis | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6687). | |||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 3 | Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl. 2002 Jun;(127):6-19. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

