Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E4GG
|
|||
| Former ID |
DCL000620
|
|||
| Drug Name |
Resatorvid
|
|||
| Synonyms |
Resatorvid; TAK-242; 243984-11-4; UNII-H2MZ648C31; CHEMBL225157; H2MZ648C31; TAK242; Resatorvid [INN]; TAK-242 (Resatorvid); C15H17ClFNO4S; SCHEMBL872197; GTPL9036; MolPort-035-773-739; BDBM50155929; ZINC13982410; 4003AH; AKOS027250774; CS-0408; KB-80786; AN-16512; HY-11109; A3850; W-5468; P111021; ethyl (6R)-6-[(2-chloro-4-fluorophenyl)sulfamoyl]cyclohexene-1-carboxylate; ethyl (6r)-6-[n-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate; ethyl (6R)-6-[(2-chloro-4-fluoro-phenyl)sulfamoyl]cyclohexene-1-carboxylate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Sepsis [ICD-11: 1G40-1G41; ICD-9: 995.91] | Phase 3 | [1] | |
| Company |
Takeda
|
|||
| Structure |
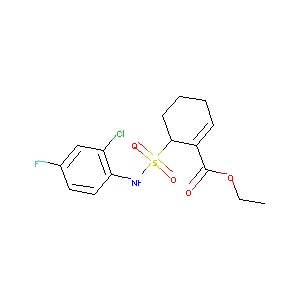 |
Download2D MOL |
||
| Formula |
C15H17ClFNO4S
|
|||
| Canonical SMILES |
CCOC(=O)C1=CCCCC1S(=O)(=O)NC2=C(C=C(C=C2)F)Cl
|
|||
| InChI |
1S/C15H17ClFNO4S/c1-2-22-15(19)11-5-3-4-6-14(11)23(20,21)18-13-8-7-10(17)9-12(13)16/h5,7-9,14,18H,2-4,6H2,1H3/t14-/m1/s1
|
|||
| InChIKey |
LEEIJTHMHDMWLJ-CQSZACIVSA-N
|
|||
| CAS Number |
CAS 243984-11-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00633477) Efficacy and Safety of Resatorvid in Patients With Sepsis-induced Cardiovascular and Respiratory Failure. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of Takeda (2009). | |||
| REF 3 | THE NOVEL SELECTIVE TOLL-LIKE RECEPTOR 4 SIGNAL TRANSDUCTION INHIBITOR TAK-242 PREVENTS ENDOTOXAEMIA IN CONSCIOUS GUINEA-PIGS. Clinical and Experimental Pharmacology and Physiology Volume 36, Issue 5-6, pages 589-593, May/June 2009 | |||
| REF 4 | TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008 Apr 14;584(1):40-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

