Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0DP8P
|
|||
| Former ID |
DNC000367
|
|||
| Drug Name |
BW755C
|
|||
| Synonyms |
BW755C; 66000-40-6; BW-755C; UNII-6V6JF56BXO; 6V6JF56BXO; CHEMBL274642; 4,5-Dihydro-1-(3-(trifluoromethyl)phenyl)-1H-pyrazol-3-amine; 1-[3-(trifluoromethyl)phenyl]-4,5-dihydro-1H-pyrazol-3-amine; 1-(3-trifluoromethylphenyl)-4,5-dihydro-1H-pyrazol-3-amine; 1-(3-Trifluoromethyl-phenyl)-4,5-dihydro-1H-pyrazol-3-ylamine; 4,5-DIHYDRO-1-[3-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-3-AMINE; 1-(3-(trifluoromethyl)phenyl)-4,5-dihydro-1H-pyrazol-3-amine; BW 755C; EINECS 266-051-8; AC1L2IRR; SCHEMBL2573191; CTK5C3353; DTXSID30216213
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Inflammation [ICD-11: 1A00-CA43.1] | Terminated | [1] | |
| Structure |
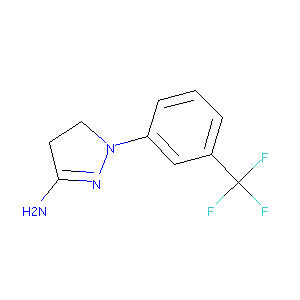 |
Download2D MOL |
||
| Formula |
C10H10F3N3
|
|||
| Canonical SMILES |
C1CN(N=C1N)C2=CC=CC(=C2)C(F)(F)F
|
|||
| InChI |
1S/C10H10F3N3/c11-10(12,13)7-2-1-3-8(6-7)16-5-4-9(14)15-16/h1-3,6H,4-5H2,(H2,14,15)
|
|||
| InChIKey |
CPXGGWXJNQSFEP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 66000-40-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
1995008, 4798294, 8180402, 12015705, 14773773, 34712751, 50070598, 50071207, 57313145, 78119914, 81656244, 91757133, 99222735, 103167028, 103857306, 103915088, 104353844, 105582736, 125695365, 127660739, 130887853, 134339811, 134349197, 135007807, 143305710, 162891670, 224097533, 225226571, 228672351, 249764864
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [2] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | BW755C, a dual lipoxygenase/cyclooxygenase inhibitor, reduces mural platelet and neutrophil deposition and vasoconstriction after angioplasty injury in pigs. J Pharmacol Exp Ther. 1996 Apr;277(1):17-21. | |||
| REF 2 | Anti-inflammatory activity of Acanthus ilicifolius. J Ethnopharmacol. 2008 Oct 30;120(1):7-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

