Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0D6ZW
|
|||
| Drug Name |
BMS-986020
|
|||
| Synonyms |
GQBRZBHEPUQRPL-LJQANCHMSA-N; 1257213-50-5; AP-3152 free acid; UNII-38CTP01B4L; 38CTP01B4L; SCHEMBL344742; GTPL9498; EX-A866; MolPort-044-616-249; ZINC113624125; AKOS030631907; CS-5844; AS-35060; HY-100619; FT-0700148; J-690107; Cyclopropanecarboxylic acid, 1-(4'-(3-methyl-4-((((1R)-1-phenylethoxy)carbonyl)amino)-5-isoxazolyl)(1,1'-biphenyl)-4-yl)-; 1-{4'-[3-Methyl-4-((R)-1-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid; 1-[4-[4-[3-methyl-4-[[(1R)-1-phenylethoxy]carbonyl
Click to Show/Hide
|
|||
| Indication | Scleroderma [ICD-11: 4A42; ICD-9: 701.0710.1] | Phase 2 | [1] | |
| Psoriasis vulgaris [ICD-11: EA90; ICD-9: 696] | Phase 1 | [2] | ||
| Company |
Bristol-Myers SquibbPrinceton, NJ
|
|||
| Structure |
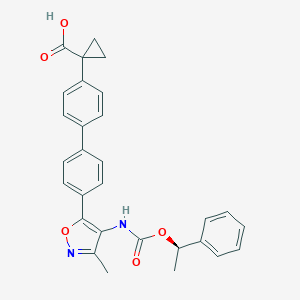 |
Download2D MOL |
||
| Formula |
C29H26N2O5
|
|||
| Canonical SMILES |
CC1=NOC(=C1NC(=O)OC(C)C2=CC=CC=C2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5(CC5)C(=O)O
|
|||
| InChI |
1S/C29H26N2O5/c1-18-25(30-28(34)35-19(2)20-6-4-3-5-7-20)26(36-31-18)23-10-8-21(9-11-23)22-12-14-24(15-13-22)29(16-17-29)27(32)33/h3-15,19H,16-17H2,1-2H3,(H,30,34)(H,32,33)/t19-/m1/s1
|
|||
| InChIKey |
GQBRZBHEPUQRPL-LJQANCHMSA-N
|
|||
| CAS Number |
CAS 1257213-50-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Lysophosphatidic acid receptor 1 (LPAR1) | Target Info | Antagonist | [2] |
| KEGG Pathway | Rap1 signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| PI3K-Akt signaling pathway | ||||
| Gap junction | ||||
| Pathways in cancer | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Pathway Interaction Database | LPA receptor mediated events | |||
| Reactome | G alpha (q) signalling events | |||
| G alpha (i) signalling events | ||||
| Lysosphingolipid and LPA receptors | ||||
| WikiPathways | Myometrial Relaxation and Contraction Pathways | |||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02588625) A Double-Blinded Study to Evaluate the Safety, Tolerability, and Efficacy of BMS-986020 Versus Placebo in Diffuse Cutaneous Systemic Sclerosis (dcSSc). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

