Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0CH7C
|
|||
| Drug Name |
Basmisanil
|
|||
| Synonyms |
1159600-41-5; UNII-788PET5SUA; 788PET5SUA; (1,1-Dioxidothiomorpholino)(6-((3-(4-fluorophenyl)-5-methylisoxazol-4-yl)methoxy)pyridin-3-yl)methanone; Basmisanil [INN]; Basmisanil [USAN:INN]; Basmisani; Basmisanil(RG1662); Basmisanil (USAN/INN); SCHEMBL2685527; CHEMBL3681419; MolPort-044-561-818; VCGRFBXVSFAGGA-UHFFFAOYSA-N; BDBM133427; EX-A1272; AKOS032947142; ZINC145814743; DB11877; CS-6046; HY-16716; (1,1-Dioxo-4-thiomorpholinyl)(6-((3-(4-fluorophenyl)-5-methylisoxazol-4-yl)methoxy)pyridin-3-yl)metha
Click to Show/Hide
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [1] | |
| Cognitive impairment [ICD-11: 6D71; ICD-10: F06.7] | Phase 2 | [2] | ||
| Complete androgen insensitivity syndrome [ICD-11: LD2A.4; ICD-10: E34.5] | Phase 2 | [3] | ||
| Immune dysregulation [ICD-11: 4A01.2; ICD-10: D89.8] | Phase 2 | [3] | ||
| Company |
Roche
|
|||
| Structure |
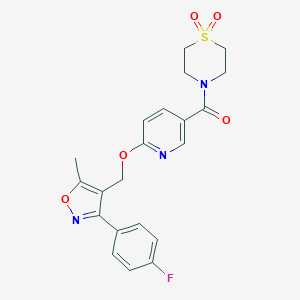 |
Download2D MOL |
||
| Formula |
C21H20FN3O5S
|
|||
| Canonical SMILES |
CC1=C(C(=NO1)C2=CC=C(C=C2)F)COC3=NC=C(C=C3)C(=O)N4CCS(=O)(=O)CC4
|
|||
| InChI |
1S/C21H20FN3O5S/c1-14-18(20(24-30-14)15-2-5-17(22)6-3-15)13-29-19-7-4-16(12-23-19)21(26)25-8-10-31(27,28)11-9-25/h2-7,12H,8-11,13H2,1H3
|
|||
| InChIKey |
VCGRFBXVSFAGGA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1159600-41-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | GABA(A) receptor alpha-5 (GABRA5) | Target Info | Modulator | [3] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Retrograde endocannabinoid signaling | ||||
| GABAergic synapse | ||||
| Morphine addiction | ||||
| Nicotine addiction | ||||
| Reactome | Ligand-gated ion channel transport | |||
| GABA A receptor activation | ||||
| WikiPathways | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||
| Iron uptake and transport | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02484703) A Study of RG1662 in Down Syndrome Among Children 6 to 11 Years of Age. | |||
| REF 2 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

