Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C9NJ
|
|||
| Former ID |
DCL000235
|
|||
| Drug Name |
STA-5326
|
|||
| Synonyms |
Apilimod; STA 5326; STA5326; N-[(3-methylphenyl)methylideneamino]-6-morpholin-4-yl-2-(2-pyridin-2-ylethoxy)pyrimidin-4-amine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Crohn disease [ICD-11: DD70; ICD-10: K50, K50.9] | Phase 2 | [1], [2] | |
| Rheumatoid arthritis [ICD-11: FA20] | Phase 2 | [1], [2] | ||
| Company |
Synta Pharma
|
|||
| Structure |
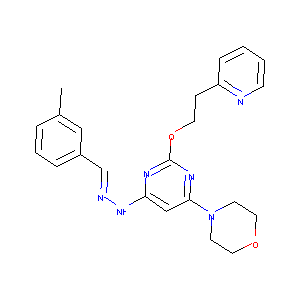 |
Download2D MOL |
||
| Formula |
C23H26N6O2
|
|||
| Canonical SMILES |
CC1=CC(=CC=C1)C=NNC2=CC(=NC(=N2)OCCC3=CC=CC=N3)N4CCOCC4
|
|||
| InChI |
1S/C23H26N6O2/c1-18-5-4-6-19(15-18)17-25-28-21-16-22(29-10-13-30-14-11-29)27-23(26-21)31-12-8-20-7-2-3-9-24-20/h2-7,9,15-17H,8,10-14H2,1H3,(H,26,27,28)/b25-17+
|
|||
| InChIKey |
HSKAZIJJKRAJAV-KOEQRZSOSA-N
|
|||
| CAS Number |
CAS 541550-19-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Interleukin-12 alpha (IL12A) | Target Info | Inhibitor | [3], [4] |
| KEGG Pathway | Cytokine-cytokine receptor interaction | |||
| Toll-like receptor signaling pathway | ||||
| RIG-I-like receptor signaling pathway | ||||
| Jak-STAT signaling pathway | ||||
| Type I diabetes mellitus | ||||
| Pertussis | ||||
| Legionellosis | ||||
| Leishmaniasis | ||||
| Chagas disease (American trypanosomiasis) | ||||
| African trypanosomiasis | ||||
| Malaria | ||||
| Toxoplasmosis | ||||
| Amoebiasis | ||||
| Tuberculosis | ||||
| Measles | ||||
| Influenza A | ||||
| Herpes simplex infection | ||||
| Inflammatory bowel disease (IBD) | ||||
| Allograft rejection | ||||
| Pathway Interaction Database | IL27-mediated signaling events | |||
| IL12-mediated signaling events | ||||
| WikiPathways | Toll-like receptor signaling pathway | |||
| Aryl Hydrocarbon Receptor Pathway | ||||
| Allograft Rejection | ||||
| Regulation of toll-like receptor signaling pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | A phase 1/2A trial of STA 5326, an oral interleukin-12/23 inhibitor, in patients with active moderate to severe Crohn's disease. Inflamm Bowel Dis. 2006 Jul;12(7):558-65. | |||
| REF 2 | Emerging drugs for moderate-to-severe psoriasis. Expert Opin Emerg Drugs. 2005 Feb;10(1):35-52. | |||
| REF 3 | Therapeutic effect of the potent IL-12/IL-23 inhibitor STA-5326 on experimental autoimmune uveoretinitis. Arthritis Res Ther. 2008;10(5):R122. | |||
| REF 4 | Selective abrogation of Th1 response by STA-5326, a potent IL-12/IL-23 inhibitor. Blood. 2007 Feb 1;109(3):1156-64. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

