Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C6GK
|
|||
| Former ID |
DNCL003456
|
|||
| Drug Name |
EPZ-5676
|
|||
| Synonyms |
pinometostat; EPZ-5676; 1380288-87-8; EPZ5676; UNII-8V9YR09EF3; UNII-F66X4M38G5; 8V9YR09EF3; CHEMBL3087499; CHEMBL3414626; F66X4M38G5; 1380288-88-9; (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]imidazol-2-yl)ethyl)cyclobutyl)(isopropyl)amino)methyl)tetrahydrofuran-3,4-diol; Pinometostat, trans-; 5'-[{cis-3-[2-(5-Tert-Butyl-1h-Benzimidazol-2-Yl)ethyl]cyclobutyl}(Propan-2-Yl)amino]-5'-Deoxyadenosine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60; ICD-9: 205] | Phase 1/2 | [1] | |
| Acute lymphoblastic leukaemia [ICD-11: 2A85; ICD-10: C85.1, C88.7] | Phase 1 | [2] | ||
| Acute lymphocytic leukaemia [ICD-11: 2B33.3; ICD-10: C91, C91.9] | Phase 1 | [3] | ||
| Company |
Epizyme
|
|||
| Structure |
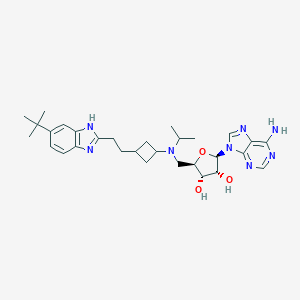 |
Download2D MOL |
||
| Formula |
C30H42N8O3
|
|||
| Canonical SMILES |
CC(C)N(CC1C(C(C(O1)N2C=NC3=C(N=CN=C32)N)O)O)C4CC(C4)CCC5=NC6=C(N5)C=C(C=C6)C(C)(C)C
|
|||
| InChI |
1S/C30H42N8O3/c1-16(2)37(13-22-25(39)26(40)29(41-22)38-15-34-24-27(31)32-14-33-28(24)38)19-10-17(11-19)6-9-23-35-20-8-7-18(30(3,4)5)12-21(20)36-23/h7-8,12,14-17,19,22,25-26,29,39-40H,6,9-11,13H2,1-5H3,(H,35,36)(H2,31,32,33)/t17?,19?,22-,25-,26-,29-/m1/s1
|
|||
| InChIKey |
LXFOLMYKSYSZQS-XKHGBIBOSA-N
|
|||
| CAS Number |
CAS 1380288-87-8
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:124919
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histone-lysine N-methyltransferase (HLNM) | Target Info | Modulator | [4] |
| KEGG Pathway | Lysine degradation | |||
| Transcriptional misregulation in cancer | ||||
| Reactome | PKMTs methylate histone lysines | |||
| WikiPathways | Histone Modifications | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03701295) Pinometostat and Azacitidine in Treating Patients With Relapsed, Refractory, or Newly Diagnosed Acute Myeloid Leukemia With 11q23 Rearrangement. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013 August 8; 122(6): 1017-1025. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

