Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C5NP
|
|||
| Former ID |
DND000036
|
|||
| Drug Name |
AGG-523
|
|||
| Synonyms |
PF-5212371, WAY-266523
Click to Show/Hide
|
|||
| Indication | Osteoarthritis [ICD-11: FA00-FA05; ICD-9: 715] | Phase 1 | [1] | |
| Company |
Pfizer
|
|||
| Structure |
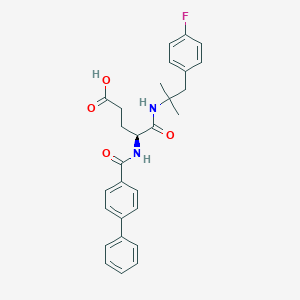 |
Download2D MOL |
||
| Formula |
C28H29FN2O4
|
|||
| Canonical SMILES |
CC(C)(CC1=CC=C(C=C1)F)NC(=O)C(CCC(=O)O)NC(=O)C2=CC=C(C=C2)C3=CC=CC=C3
|
|||
| InChI |
1S/C28H29FN2O4/c1-28(2,18-19-8-14-23(29)15-9-19)31-27(35)24(16-17-25(32)33)30-26(34)22-12-10-21(11-13-22)20-6-4-3-5-7-20/h3-15,24H,16-18H2,1-2H3,(H,30,34)(H,31,35)(H,32,33)/t24-/m0/s1
|
|||
| InChIKey |
JWQMTWCFNZSLNR-DEOSSOPVSA-N
|
|||
| CAS Number |
CAS 920289-29-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aggrecanase (ADAMTS5) | Target Info | Inhibitor | [2] |
| Reactome | Degradation of the extracellular matrix | |||
| O-glycosylation of TSR domain-containing proteins | ||||
| WikiPathways | Endochondral Ossification | |||
| Vitamin D Receptor Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00454298) Study Evaluating AGG-523 in Subjects With Severe Osteoarthritis Requiring Total Knee Replacement. U.S. National Institutes of Health. | |||
| REF 2 | Elevated aggrecanase activity in a rat model of joint injury is attenuated by an aggrecanase specific inhibitor. Osteoarthritis Cartilage. 2011 Mar;19(3):315-23. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

