Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0B6YB
|
|||
| Former ID |
DIB003830
|
|||
| Drug Name |
LE-SN38
|
|||
| Synonyms |
7-Ethyl-10-hydroxycamptothecin; 86639-52-3; SN-38; SN 38 lactone; 7-Ethyl-10-hydroxy-camptothecin; SN 38; 10-Hydroxy-7-ethylcamptothecin; 7-Ethyl-10-hydroxycamptothecine; SN38; 7-Ethyl-10-hydroxy-20(S)-camptothecin; UNII-0H43101T0J; Captothecin, 7-ethyl-10-hydroxy-; CHEBI:8988; (S)-4,11-Diethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; 7-Ethyl-10-hydroxy camptothecin; NSC673596; 0H43101T0J; (+)-7-ETHYL-10-HYDROXYCAMPTOTHECIN; 113015-38-6
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Colorectal cancer [ICD-11: 2B91.Z] | Phase 2 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [1], [2] | ||
| Company |
NeoPharm
|
|||
| Structure |
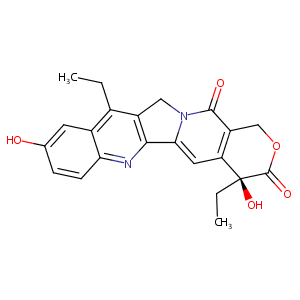 |
Download2D MOL |
||
| Formula |
C22H20N2O5
|
|||
| Canonical SMILES |
CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)C4(CC)O)C2=NC5=C1C=C(C=C5)O
|
|||
| InChI |
1S/C22H20N2O5/c1-3-12-13-7-11(25)5-6-17(13)23-19-14(12)9-24-18(19)8-16-15(20(24)26)10-29-21(27)22(16,28)4-2/h5-8,25,28H,3-4,9-10H2,1-2H3/t22-/m0/s1
|
|||
| InChIKey |
FJHBVJOVLFPMQE-QFIPXVFZSA-N
|
|||
| CAS Number |
CAS 86639-52-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
13355, 515301, 622834, 10233636, 11430409, 12012598, 14805294, 14805295, 44434437, 49835447, 50028359, 50112971, 56311337, 56311445, 56311571, 56312630, 57337873, 71831605, 80486942, 87559919, 89360249, 92712502, 103220117, 103996165, 104373061, 126588659, 126625532, 126656661, 126666539, 127855413, 134340412, 134340540, 135062353, 135693781, 135710073, 136377612, 136897516, 136911131, 137138435, 142309355, 144115510, 152059852, 152164367, 152200177, 152245349, 162178284, 162223790, 163367112, 164178264, 164194982
|
|||
| ChEBI ID |
CHEBI:8988
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [3] | |||
| Resulting Metabolite | SN-38G; cyanuric acid | |||
| Metabolic Effect | Decrease activity | |||
| Description | SN-38 can be metabolized to SN-38G and cyanuric acid by gut microbiota, which results in the decrease of the drug's activity. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase I (TOP1) | Target Info | Inhibitor | [4], [5] |
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | DNA replication | |||
| Pathway Interaction Database | Caspase Cascade in Apoptosis | |||
| WikiPathways | Integrated Pancreatic Cancer Pathway | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Randomized phase 2 study of pegylated SN-38 (EZN-2208) or irinotecan plus cetuximab in patients with advanced colorectal cancer. Cancer. 2013 Dec 15;119(24):4223-30. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6925). | |||
| REF 3 | The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. 2016 Apr;14(5):273-87. | |||
| REF 4 | Analysis of type of cell death induced by topoisomerase inhibitor SN-38 in human oral squamous cell carcinoma cell lines. Anticancer Res. 2012 Nov;32(11):4823-32. | |||
| REF 5 | J Clin Oncol (Meeting Abstracts) May 2011 vol. 29 no. 15_suppl 7079 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

