Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0B5FZ
|
|||
| Former ID |
DAP001463
|
|||
| Drug Name |
Cefozopran
|
|||
| Synonyms |
CZOP; Cefozopran [INN]; SCE 2787; Cefozopran (INN); (-)-1-(((6R,7R)-7-(2-(5-Amino-1,2,4-thiadiazol-3-yl)glyoxylamido)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1H-imidazo(1,2-b)pyridazin-4-ium hydroxide inner salt, 7(sup 2)-(Z)-(O-methyloxime); Imidazo(1,2-b)pyridazinium, 1-((6R,7R)-7-(((2Z)-(5-amino-1,2,4-thiadiazol-3-yl)(methoxyimino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-, inner salt
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Gram-positive bacterial infection [ICD-11: 1B74-1G40] | Approved | [1] | |
| Company |
Takeda
|
|||
| Structure |
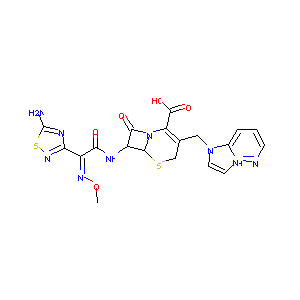 |
Download2D MOL |
||
| Formula |
C19H17N9O5S2
|
|||
| Canonical SMILES |
CON=C(C1=NSC(=N1)N)C(=O)NC2C3N(C2=O)C(=C(CS3)C[N+]4=C5C=CC=NN5C=C4)C(=O)[O-]
|
|||
| InChI |
1S/C19H17N9O5S2/c1-33-24-11(14-23-19(20)35-25-14)15(29)22-12-16(30)28-13(18(31)32)9(8-34-17(12)28)7-26-5-6-27-10(26)3-2-4-21-27/h2-6,12,17H,7-8H2,1H3,(H3-,20,22,23,25,29,31,32)/b24-11-/t12-,17-/m1/s1
|
|||
| InChIKey |
QDUIJCOKQCCXQY-WHJQOFBOSA-N
|
|||
| CAS Number |
CAS 113359-04-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:3502
|
|||
| SuperDrug ATC ID |
J01DE03
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Dihydropteroate synthetase (Bact folP) | Target Info | Inhibitor | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Emerging drugs for bacterial urinary tract infections. Expert Opin Emerg Drugs. 2005 May;10(2):275-98. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

