Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A9PN
|
|||
| Former ID |
DIB015436
|
|||
| Drug Name |
GDC-0853
|
|||
| Synonyms |
Fenebrutinib
Click to Show/Hide
|
|||
| Indication | Multiple sclerosis [ICD-11: 8A40] | Phase 3 | [1] | |
| Chronic idiopathic urticaria [ICD-11: EB00.1; ICD-10: L50.1; ICD-9: 708] | Phase 2 | [2] | ||
| Autoimmune disease [ICD-11: 4A40-4A45; ICD-9: 279] | Phase 1 | [3] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [4] | ||
| Company |
GenentechSouth San Francisco, CA
|
|||
| Structure |
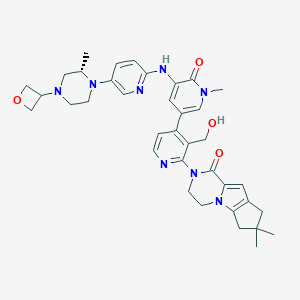 |
Download2D MOL |
||
| Formula |
C37H44N8O4
|
|||
| Canonical SMILES |
CC1CN(CCN1C2=CN=C(C=C2)NC3=CC(=CN(C3=O)C)C4=C(C(=NC=C4)N5CCN6C7=C(CC(C7)(C)C)C=C6C5=O)CO)C8COC8
|
|||
| InChI |
1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1
|
|||
| InChIKey |
WNEODWDFDXWOLU-QHCPKHFHSA-N
|
|||
| CAS Number |
CAS 1434048-34-6
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04586023) Study To Evaluate The Efficacy And Safety Of Fenebrutinib Compared With Teriflunomide In Relapsing Multiple Sclerosis (RMS) (FENhance). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | ClinicalTrials.gov (NCT01991184) A Study of GDC-0853 in Patients With Resistant B-Cell Lymphoma or Chronic Lymphocytic Leukemia.. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

